Copolymerization of lactones and bioaromatics via concurrent ring-opening polymerization/polycondensation
2017, Green Chemistry

Sign up for access to the world's latest research
Abstract
Concurrent ring-opening polymerization/polycondensation of lactones and bioaromatic hydroxy-acids yields random copolymers with improved and controlled thermal properties.





![Figure S1. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 1).](https://figures.academia-assets.com/97857879/figure_003.jpg)
![Figure 82. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 2).](https://figures.academia-assets.com/97857879/figure_004.jpg)
![Figure S3. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 3).](https://figures.academia-assets.com/97857879/figure_005.jpg)
![Figure S4. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 4).](https://figures.academia-assets.com/97857879/figure_006.jpg)
![Figure S5. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 5).](https://figures.academia-assets.com/97857879/figure_007.jpg)
![Figure S6. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 6).](https://figures.academia-assets.com/97857879/figure_008.jpg)
![Figure $7. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 7).](https://figures.academia-assets.com/97857879/figure_009.jpg)
![Figure S8. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 8).](https://figures.academia-assets.com/97857879/figure_010.jpg)
![Figure S9. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 8 and Table S2 entry 9).](https://figures.academia-assets.com/97857879/figure_011.jpg)

![Figure S11. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [90:10] (Table S2, entry 2)](https://figures.academia-assets.com/97857879/figure_013.jpg)
![Figure S12. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [80:20] (Table S2, entry 3).](https://figures.academia-assets.com/97857879/figure_014.jpg)
![Figure S13. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [70:30] (Table S2, entry 4)](https://figures.academia-assets.com/97857879/figure_015.jpg)
![Figure § S14. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [60:40] (Table S2, entry 5).](https://figures.academia-assets.com/97857879/figure_016.jpg)
![Figure $15. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] (Table S2, entry 6)](https://figures.academia-assets.com/97857879/figure_017.jpg)
![Figure S16. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [40:60] (Table S2, entry 7)](https://figures.academia-assets.com/97857879/figure_018.jpg)
![Figure S17. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [30:70] (Table S2, entry 8).](https://figures.academia-assets.com/97857879/figure_019.jpg)
![Figure $18. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [10:90] (Table S2, entry 10).](https://figures.academia-assets.com/97857879/figure_020.jpg)

![Figure $20. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [90:10] (Table S2, entry 12). Figure S19. GPC Chromatogram of polyethylene syringate (Table S2, entry 21).](https://figures.academia-assets.com/97857879/figure_022.jpg)
![Figure | $21. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [80:20] (Table S2, entry 13)](https://figures.academia-assets.com/97857879/figure_023.jpg)
![Figure $22. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [70:30] (Table 2, entry 14).](https://figures.academia-assets.com/97857879/figure_024.jpg)
![Figure $23. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [60:40] (Table S2, entry 15).](https://figures.academia-assets.com/97857879/figure_025.jpg)

![Figure $25. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [40:60] (Table S2, entry 17).](https://figures.academia-assets.com/97857879/figure_027.jpg)
![Figure | $26. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [30:70] (Table S2, entry 18).](https://figures.academia-assets.com/97857879/figure_028.jpg)
![Figure S27. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [20:80] (Table S2, entry 19](https://figures.academia-assets.com/97857879/figure_029.jpg)
![Figure $28. GPC Chromatogram of copoly(caprolactone/ hydroxyethylvanilic acid) [10:90] (Table S2, entry 20).](https://figures.academia-assets.com/97857879/figure_030.jpg)
![Figure S29. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [90:10] (Table S2, entry 22).](https://figures.academia-assets.com/97857879/figure_031.jpg)

![Figure | $31. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [70:30] (Table S2, entry 24).](https://figures.academia-assets.com/97857879/figure_033.jpg)
![Figure $32. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [60:40] (Table S2, entry 25).](https://figures.academia-assets.com/97857879/figure_034.jpg)
![Figure | $33. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [50:50] (Table S2, entry 26)](https://figures.academia-assets.com/97857879/figure_035.jpg)
![Figure S34. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [40:60] (Table S2, entry 27).](https://figures.academia-assets.com/97857879/figure_036.jpg)
![Figure $35. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [30:70] (Table S2, entry 28)](https://figures.academia-assets.com/97857879/figure_037.jpg)
![Figure $36. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [20:80] (Table S2, entry 29).](https://figures.academia-assets.com/97857879/figure_038.jpg)
![Figure | S837. GPC Chromatogram of copoly(caprolactone/ hydroxyethylferulic acid) [10:90] (Table S2, entry 30).](https://figures.academia-assets.com/97857879/figure_039.jpg)

![Figure S39. GPC Chromatogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [80:20] (Table S2, entry 32)](https://figures.academia-assets.com/97857879/figure_041.jpg)


![Figure S41. GPC Chromatogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [20:80] (Table S2, entry 34)](https://figures.academia-assets.com/97857879/figure_044.jpg)
![Figure $43. GPC Chromatogram of copoly(L-lactide/ hydroxyethylsyringic acid) [80:20] (Table S3, entry 2).](https://figures.academia-assets.com/97857879/figure_045.jpg)

![Figure S45. GPC Chromatogram of copoly(L-lactide/ hydroxyethylsyringic acid) [40:60] (Table S3, entry 4).](https://figures.academia-assets.com/97857879/figure_047.jpg)
![Figure S46. GPC Chromatogram of copoly(L-lactide/ hydroxyethylsyringic acid) [20:80] (Table S3, entry 5).](https://figures.academia-assets.com/97857879/figure_048.jpg)
![Figure S47. GPC Chromatogram of copoly(L-lactide/ hydroxyethylvanillic acid) [80:20] (Table S3, entry 7).](https://figures.academia-assets.com/97857879/figure_049.jpg)

![Figure S49. GPC Chromatogram of copoly(L-lactide/ hydroxyethylvanillic acid) [40:60] (Table S3, entry 9).](https://figures.academia-assets.com/97857879/figure_051.jpg)

![Figure S51. GPC Chromatogram of copoly(L-lactide/ hydroxyethylferulic acid) [90:10] (Table S3, entry 12).](https://figures.academia-assets.com/97857879/figure_053.jpg)

![Figure S53. GPC Chromatogram of copoly(L-lactide/ hydroxyethylferulic acid) [70:30] (Table S3, entry 14).](https://figures.academia-assets.com/97857879/figure_055.jpg)


![Figure S56. GPC Chromatogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [90:10] (Table S3, entry 18) Figure S55. GPC Chromatogram of copoly(L-lactide/ hydroxyethylferulic acid) [20:80] (Table S3, entry 16).](https://figures.academia-assets.com/97857879/figure_058.jpg)

![Figure S58. GPC Chromatogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [70:30] (Table S3, entry 20).](https://figures.academia-assets.com/97857879/figure_060.jpg)
![Figure S59. GPC Chromatogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [60:40] (Table S3, entry 21).](https://figures.academia-assets.com/97857879/figure_061.jpg)

![‘igure $61. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 min. (Table S4, entry 1).](https://figures.academia-assets.com/97857879/figure_063.jpg)
![Figure S62. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 20 min. (Table S4, entry 2).](https://figures.academia-assets.com/97857879/figure_064.jpg)
![‘igure. $63. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 30 min. (Table S4, entry 3).](https://figures.academia-assets.com/97857879/figure_065.jpg)
![Figure S64. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1 hour (Table S4, entry 4)](https://figures.academia-assets.com/97857879/figure_066.jpg)
![Figure S65. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1.5 hours (Table S4, entr 5).](https://figures.academia-assets.com/97857879/figure_067.jpg)
![Figure S66. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2 hours (Table S4, entry 6).](https://figures.academia-assets.com/97857879/figure_068.jpg)
![Figure S67. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2.5 hours (Table S4, entry 7).](https://figures.academia-assets.com/97857879/figure_069.jpg)
![Figure | S68. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3 hours (Table S4, entry 8).](https://figures.academia-assets.com/97857879/figure_070.jpg)
![Figure S69. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3.5 hours (Table S4, entry 9).](https://figures.academia-assets.com/97857879/figure_071.jpg)
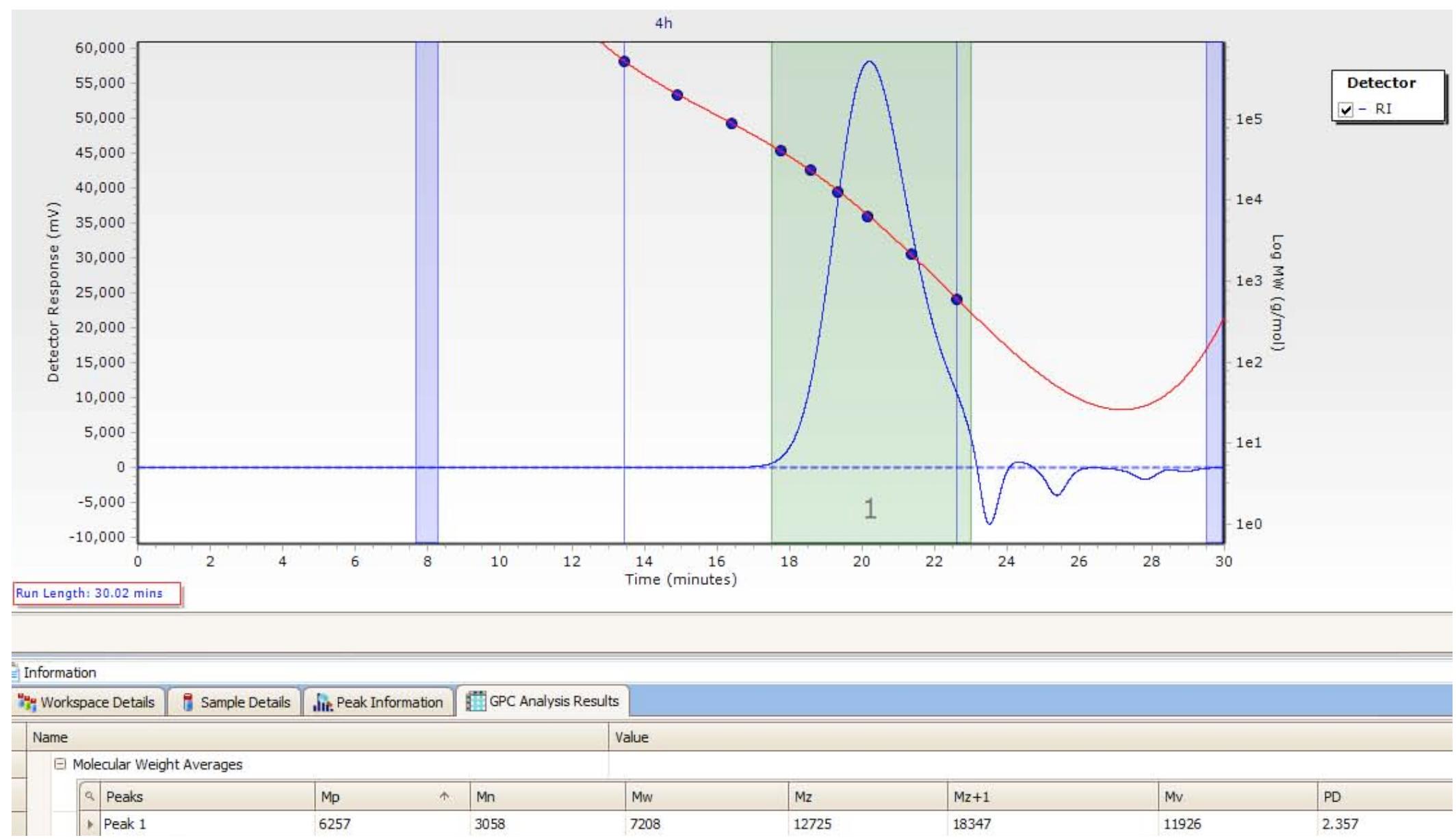
![Figure S71. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4.5 hours (Table S4, entr 11).](https://figures.academia-assets.com/97857879/figure_073.jpg)
![Figure $72. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 5 hours (Table S4, entry 12).](https://figures.academia-assets.com/97857879/figure_074.jpg)
![Figure S73. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 6 hours (Table S4, entry 13](https://figures.academia-assets.com/97857879/figure_075.jpg)
![Figure S74. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 7 hours (Table S4, entry 14).](https://figures.academia-assets.com/97857879/figure_076.jpg)
![‘igure S75. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 8 hours (Table S4, entry 15).](https://figures.academia-assets.com/97857879/figure_077.jpg)
![Figure S76. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 9 hours (Table S4, entry 16)](https://figures.academia-assets.com/97857879/figure_078.jpg)
![Figure S77 . GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 hours (Table S4, entry 17).](https://figures.academia-assets.com/97857879/figure_079.jpg)
![Figure $78. GPC Chromatogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 13 hours (Table S4, entr 18).](https://figures.academia-assets.com/97857879/figure_080.jpg)
![Figure S79. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 1).](https://figures.academia-assets.com/97857879/figure_081.jpg)

![Figure S81. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 3).](https://figures.academia-assets.com/97857879/figure_083.jpg)

![Figure S82. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 5).](https://figures.academia-assets.com/97857879/figure_085.jpg)


![Figure S84. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 7). Figure S83. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 6).](https://figures.academia-assets.com/97857879/figure_088.jpg)
![Figure S85. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 8).](https://figures.academia-assets.com/97857879/figure_089.jpg)
![Figure S86. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S1, entry 9 and Table S2. entry 9).](https://figures.academia-assets.com/97857879/figure_090.jpg)


![Figure S88. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [90:10] (Table S2, entry 2).](https://figures.academia-assets.com/97857879/figure_093.jpg)
![Figure S90. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [70:30] (Table S2, entry 4).](https://figures.academia-assets.com/97857879/figure_094.jpg)




![Figure S92. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] (Table S2, entry 6).](https://figures.academia-assets.com/97857879/figure_099.jpg)
![Figure $93. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [40:60] (Table S2, entry 7).](https://figures.academia-assets.com/97857879/figure_100.jpg)

![Figure S94. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [30:70] (Table S2, entry 8).](https://figures.academia-assets.com/97857879/figure_102.jpg)

![Figure S95. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [10:90] (Table S2, entry 10).](https://figures.academia-assets.com/97857879/figure_104.jpg)
![Figure S97. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [90:10] (Table S2, entry 12).](https://figures.academia-assets.com/97857879/figure_105.jpg)
![Figure S98. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [80:20] (Table S2, entry 13).](https://figures.academia-assets.com/97857879/figure_106.jpg)
![Figure S99. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [70:30] (Table S2, entry 14).](https://figures.academia-assets.com/97857879/figure_107.jpg)

![Figure $101. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [50:50] (Table S2, entry 16).](https://figures.academia-assets.com/97857879/figure_109.jpg)
![Figure $102. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [40:60] (Table S2, entry 17).](https://figures.academia-assets.com/97857879/figure_110.jpg)
![Figure $103. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [30:70] (Table S2, entry 18).](https://figures.academia-assets.com/97857879/figure_111.jpg)
![Figure $104. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [20:80] (Table S2, entry 19).](https://figures.academia-assets.com/97857879/figure_112.jpg)
![Figure $105. DSC Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [10:90] (Table S2, entry 20).](https://figures.academia-assets.com/97857879/figure_113.jpg)


![Figure $108. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [80:20] (Table S2, entry 23). Figure $107. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [90:10] (Table $2, entry 22).](https://figures.academia-assets.com/97857879/figure_116.jpg)
![Figure $109. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [70:30] (Table S2, entry 24).](https://figures.academia-assets.com/97857879/figure_117.jpg)
![Figure $110. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [60:40] (Table S2, entry 25).](https://figures.academia-assets.com/97857879/figure_118.jpg)

![Figure $112. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [40:60] (Table S2, entry 27). Figure S111. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [50:50] (Table S2, entry 26).](https://figures.academia-assets.com/97857879/figure_120.jpg)

![Figure $114. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [20:80] (Table S2, entry 29). Figure $113. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [30:70] (Table S2, entry 28).](https://figures.academia-assets.com/97857879/figure_122.jpg)
![Figure $115. DSC Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [10:90] (Table S2, entry 30).](https://figures.academia-assets.com/97857879/figure_123.jpg)


![Figure $118. DSC Thermogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [50:50] (Table S2, entry 33). Figure $117. DSC Thermogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [80:20] (Table S2, entry 32).](https://figures.academia-assets.com/97857879/figure_126.jpg)

![Figure $119. DSC Thermogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [20:80] (Table S2, entry 34).](https://figures.academia-assets.com/97857879/figure_128.jpg)


![Figure $122. DSC Thermogram of copoly(L-lactide/ hydroxyethylsyringic acid) [80:20] (Table S3, entry 2).](https://figures.academia-assets.com/97857879/figure_131.jpg)
![Figure $123. DSC Thermogram of copoly(L-lactide/ hydroxyethylsyringic acid) [60:40] (Table S3, entry 3).](https://figures.academia-assets.com/97857879/figure_132.jpg)

![Figure S125. DSC Thermogram of copoly(L-lactide/ hydroxyethylsyringic acid) [20:80] (Table S3, entry 5).](https://figures.academia-assets.com/97857879/figure_134.jpg)
![Figure $126. DSC Thermogram of copoly(L-lactide/ hydroxyethylvanillic acid) [80:20] (Table S3, entry 7).](https://figures.academia-assets.com/97857879/figure_135.jpg)
![Figure $127. DSC Thermogram of copoly(L-lactide/ hydroxyethylvanillic acid) [60:40] (Table $3, entry 8).](https://figures.academia-assets.com/97857879/figure_136.jpg)

![Figure $129. DSC Thermogram of copoly(L-lactide/ hydroxyethylvanillic acid) [20:80] (Table S3, entry 9).](https://figures.academia-assets.com/97857879/figure_138.jpg)


![Figure $132. DSC Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [70:30] (Table S3, entry 14). Figure $131. DSC Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [80:20] (Table S3, entry 13).](https://figures.academia-assets.com/97857879/figure_141.jpg)
![Figure $133. DSC Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [60:40] (Table S3, entry 15).](https://figures.academia-assets.com/97857879/figure_142.jpg)


![Figure $136. DSC Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [80:20] (Table S3, entry 19). Figure $135. DSC Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [90:10] (Table $3, entry 18).](https://figures.academia-assets.com/97857879/figure_145.jpg)
![Figure $137. DSC Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [70:30] (Table S3, entry 20).](https://figures.academia-assets.com/97857879/figure_146.jpg)
![Figure $138. DSC Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [60:40] (Table S3, entry 21).](https://figures.academia-assets.com/97857879/figure_147.jpg)
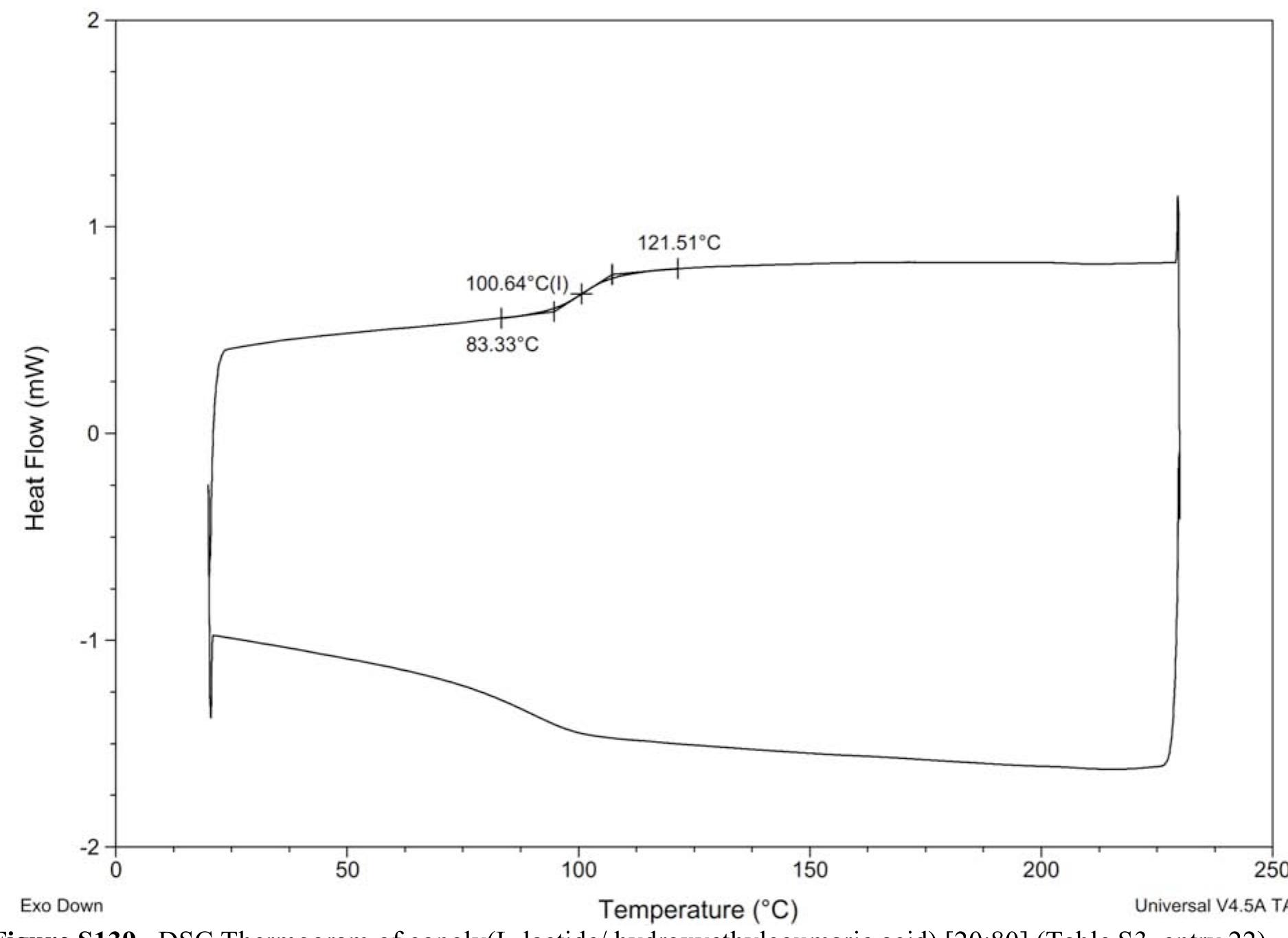
![Figure $140. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 min. (Table S4, entry 1). Figure S139. DSC Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [20:80] (Table S3, entry 22).](https://figures.academia-assets.com/97857879/figure_149.jpg)
![Figure $141. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 20 min. (Table S4, entry 2).](https://figures.academia-assets.com/97857879/figure_150.jpg)
![Figure $8142. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 30 min. (Table S4, entry 3).](https://figures.academia-assets.com/97857879/figure_151.jpg)
![Figure $143. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1 hour (Table S4, entry 4).](https://figures.academia-assets.com/97857879/figure_152.jpg)
![Figure $144. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1.5 hours (Table S4, entr 5).](https://figures.academia-assets.com/97857879/figure_153.jpg)
![Figure $145. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2 hours (Table S4, entry 6).](https://figures.academia-assets.com/97857879/figure_154.jpg)
![Figure S146. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2.5 hours (Table S4, entry 7).](https://figures.academia-assets.com/97857879/figure_155.jpg)


![Figure S148. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3.5 hours (Table S4, entry 0) Figure $147. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3 hours (Table S4, entry 8).](https://figures.academia-assets.com/97857879/figure_158.jpg)
![ee ee: eee Figure $149. DSC Thermogram of copoly(caprolactone/ ‘hydroxyethylsyringic acid) [50:50] at 4 hours (Table S4, entry 10).](https://figures.academia-assets.com/97857879/figure_159.jpg)

![Figure $150. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4.5 hours (Table $4, entry 11).](https://figures.academia-assets.com/97857879/figure_161.jpg)

![Figure S152. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 6 hours (Table S4, entry 13). Figure $151. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 5 hours (Table S4, entry 12).](https://figures.academia-assets.com/97857879/figure_163.jpg)
![Figure $153. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 7 hours (Table S4, entry 14).](https://figures.academia-assets.com/97857879/figure_164.jpg)
![Figure $154. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 8 hours (Table S4, entry 15).](https://figures.academia-assets.com/97857879/figure_165.jpg)
![Re ee Figure $155. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 9 hours (Table S4, entry 16)](https://figures.academia-assets.com/97857879/figure_166.jpg)
![Figure $156. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 hours (Table S4, entr 17).](https://figures.academia-assets.com/97857879/figure_167.jpg)
![Figure $157. DSC Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 13 hours (Table S4, entry 18).](https://figures.academia-assets.com/97857879/figure_168.jpg)


![Figure $159. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [90:10] (Table S2, entry 2).](https://figures.academia-assets.com/97857879/figure_171.jpg)
![Figure S160. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [80:20] (Table S2, entry 3).](https://figures.academia-assets.com/97857879/figure_172.jpg)

![Figure $162. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [60:40] (Table S2, entry 5).](https://figures.academia-assets.com/97857879/figure_174.jpg)

![Figure S163. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] (Table S2, entry 6).](https://figures.academia-assets.com/97857879/figure_176.jpg)
![Figure $164. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [40:60] (Table S2, entry 7).](https://figures.academia-assets.com/97857879/figure_177.jpg)
![Figure $165. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [30:70] (Table S2, entry 8).](https://figures.academia-assets.com/97857879/figure_178.jpg)
![Figure $166. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S2, entry 9).](https://figures.academia-assets.com/97857879/figure_179.jpg)
![Figure S167. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [10:90] (Table S2, entry 10).](https://figures.academia-assets.com/97857879/figure_180.jpg)

![Figure $169. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [90:10] (Table S2, entry 12).](https://figures.academia-assets.com/97857879/figure_182.jpg)
![Figure $170. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [80:20] (Table S2, entry 13).](https://figures.academia-assets.com/97857879/figure_183.jpg)
![Figure $171. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [70:30] (Table S2, entry 14).](https://figures.academia-assets.com/97857879/figure_184.jpg)
![Figure $172. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [60:40] (Table S2, entry 15).](https://figures.academia-assets.com/97857879/figure_185.jpg)

![Figure $174. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [40:60] (Table S2, entry 17).](https://figures.academia-assets.com/97857879/figure_187.jpg)
![Figure $175. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [30:70] (Table S2, entry 18).](https://figures.academia-assets.com/97857879/figure_188.jpg)
![Figure S176. TGA Thermogram of copoly(caprolactone/ hydroxyethylvanillic acid) [20:80] (Table S2, entry 19).](https://figures.academia-assets.com/97857879/figure_189.jpg)


![Figure $179. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [90:10] (Table S2, entry 22).](https://figures.academia-assets.com/97857879/figure_192.jpg)
![Figure S180. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [80:20] (Table S2, entry 23).](https://figures.academia-assets.com/97857879/figure_193.jpg)
![Figure $181. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [70:30] (Table S2, entry 24).](https://figures.academia-assets.com/97857879/figure_194.jpg)
![Figure $182. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [60:40] (Table S2, entry 25).](https://figures.academia-assets.com/97857879/figure_195.jpg)
![Figure $183. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [50:50] (Table S2, entry 26).](https://figures.academia-assets.com/97857879/figure_196.jpg)
![Figure S184. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [40:60] (Table S3, entry 37).](https://figures.academia-assets.com/97857879/figure_197.jpg)
![Figure $185. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [30:70] (Table S2, entry 28).](https://figures.academia-assets.com/97857879/figure_198.jpg)
![Figure $186. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [20:80] (Table S2, entry 29).](https://figures.academia-assets.com/97857879/figure_199.jpg)
![Figure 8187. TGA Thermogram of copoly(caprolactone/ hydroxyethylferulic acid) [10:90] (Table S2, entry 30).](https://figures.academia-assets.com/97857879/figure_200.jpg)


![Figure $189. TGA Thermogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [80:20] (Table S2, entry 32). Figure S188. TGA Thermogram of polyethylene ferulate (Table 82, entry 31).](https://figures.academia-assets.com/97857879/figure_203.jpg)
![Figure 8190. TGA Thermogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [50:50] (Table S2, entry 33).](https://figures.academia-assets.com/97857879/figure_204.jpg)
![Figure $191. TGA Thermogram of copoly(caprolactone/ hydroxyethylcoumaric acid) [20:80] (Table S2, entry 34).](https://figures.academia-assets.com/97857879/figure_205.jpg)


![tigure $194. TGA Thermogram of copoly(L-lactide/ hydroxyethylsyringic acid) [80:20] (Table $3, entry 2).](https://figures.academia-assets.com/97857879/figure_208.jpg)

![Figure $196. TGA Thermogram of copoly(L-lactide/ hydroxyethylsyringic acid) [40:60] (Table $3, entry 4).](https://figures.academia-assets.com/97857879/figure_210.jpg)

![Figure S198. TGA Thermogram of copoly(L-lactide/ hydroxyethylvanillic acid) [80:20] (Table $3, entry 7).](https://figures.academia-assets.com/97857879/figure_212.jpg)
![Figure $199. TGA Thermogram of copoly(L-lactide/ hydroxyethylvanillic acid) [60:40] (Table S3, entry 8).](https://figures.academia-assets.com/97857879/figure_213.jpg)
![Figure S200. TGA Thermogram of copoly(L-lactide/ hydroxyethylvanillic acid) [40:60] (Table S3, entry 9).](https://figures.academia-assets.com/97857879/figure_214.jpg)

![Figure $202. TGA Thermogram of copoly(L-lactide/ hydroxyethyl ferulic acid) [90:10] (Table $3, entry 12).](https://figures.academia-assets.com/97857879/figure_216.jpg)
![Figure $203. TGA Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [80:20] (Table $3, entry 13).](https://figures.academia-assets.com/97857879/figure_217.jpg)
![Figure $204. TGA Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [70:30] (Table $3, entry 14).](https://figures.academia-assets.com/97857879/figure_218.jpg)
![Figure S205. TGA Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [60:40] (Table S3, entry 15).](https://figures.academia-assets.com/97857879/figure_219.jpg)
![Figure S206. TGA Thermogram of copoly(L-lactide/ hydroxyethylferulic acid) [20:80] (Table S3, entry 16).](https://figures.academia-assets.com/97857879/figure_220.jpg)

![Figure S208. TGA Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [80:20] (Table S3, entry 19).](https://figures.academia-assets.com/97857879/figure_222.jpg)
![Figure S209. TGA Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [70:30] (Table S3, entry 20).](https://figures.academia-assets.com/97857879/figure_223.jpg)
![Figure 8210. TGA Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [60:40] (Table S3, entry 21).](https://figures.academia-assets.com/97857879/figure_224.jpg)
![Figure S211. TGA Thermogram of copoly(L-lactide/ hydroxyethylcoumaric acid) [20:80] (Table S3, entry 22).](https://figures.academia-assets.com/97857879/figure_225.jpg)
![Figure $8212. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 min. (Table S4, entry 1).](https://figures.academia-assets.com/97857879/figure_226.jpg)

![Figure 8214. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 30 min. (Table S4, entry 3).](https://figures.academia-assets.com/97857879/figure_228.jpg)

![Figure S216. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1.5 hours (Table S4, entry 5).](https://figures.academia-assets.com/97857879/figure_230.jpg)

![Figure $218. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2.5 hours (Table S4, entry 7).](https://figures.academia-assets.com/97857879/figure_232.jpg)

![Figure $8220. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3.5 hours (Table S4, entry 9).](https://figures.academia-assets.com/97857879/figure_234.jpg)

![Figure $222. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4.5 hours (Table S4, entry 11).](https://figures.academia-assets.com/97857879/figure_236.jpg)

![Figure $224. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 6 hours (Table S4, entry 13)](https://figures.academia-assets.com/97857879/figure_238.jpg)
![Figure 8225. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 7 hours (Table S4, entry 14).](https://figures.academia-assets.com/97857879/figure_239.jpg)
![Figure 8226. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 8 hours (Table S4, entry 15).](https://figures.academia-assets.com/97857879/figure_240.jpg)
![Figure 8227. TGA Thermogram of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 9 hours (Table S4, entry 16).](https://figures.academia-assets.com/97857879/figure_241.jpg)
![ere wee AES we gay Figure $228. TGA Thermogram of copoly(caprolactone/ ‘hydroxyethylsyringic acid) [50:50] at 10 hours (Table S4, entry 17).](https://figures.academia-assets.com/97857879/figure_242.jpg)


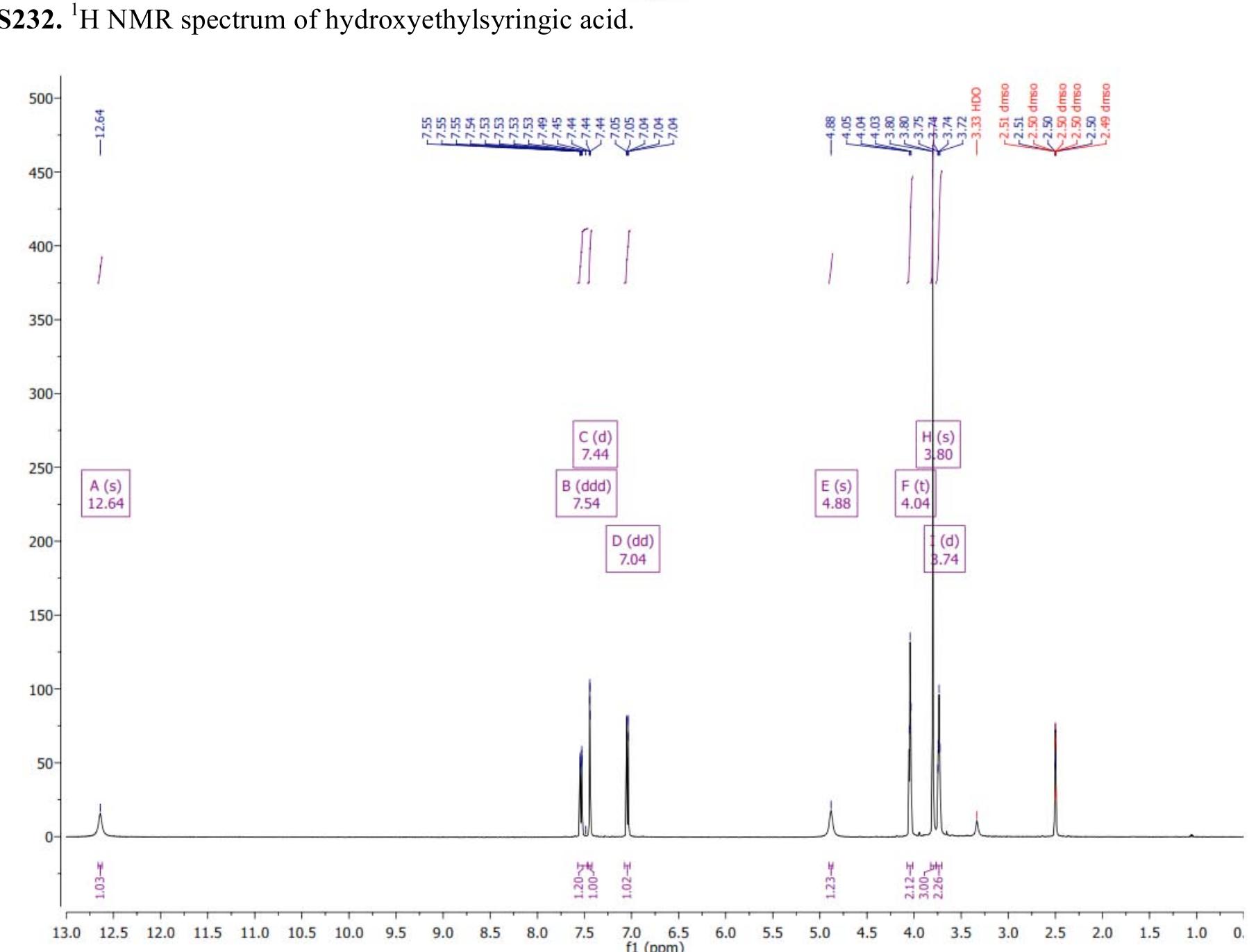
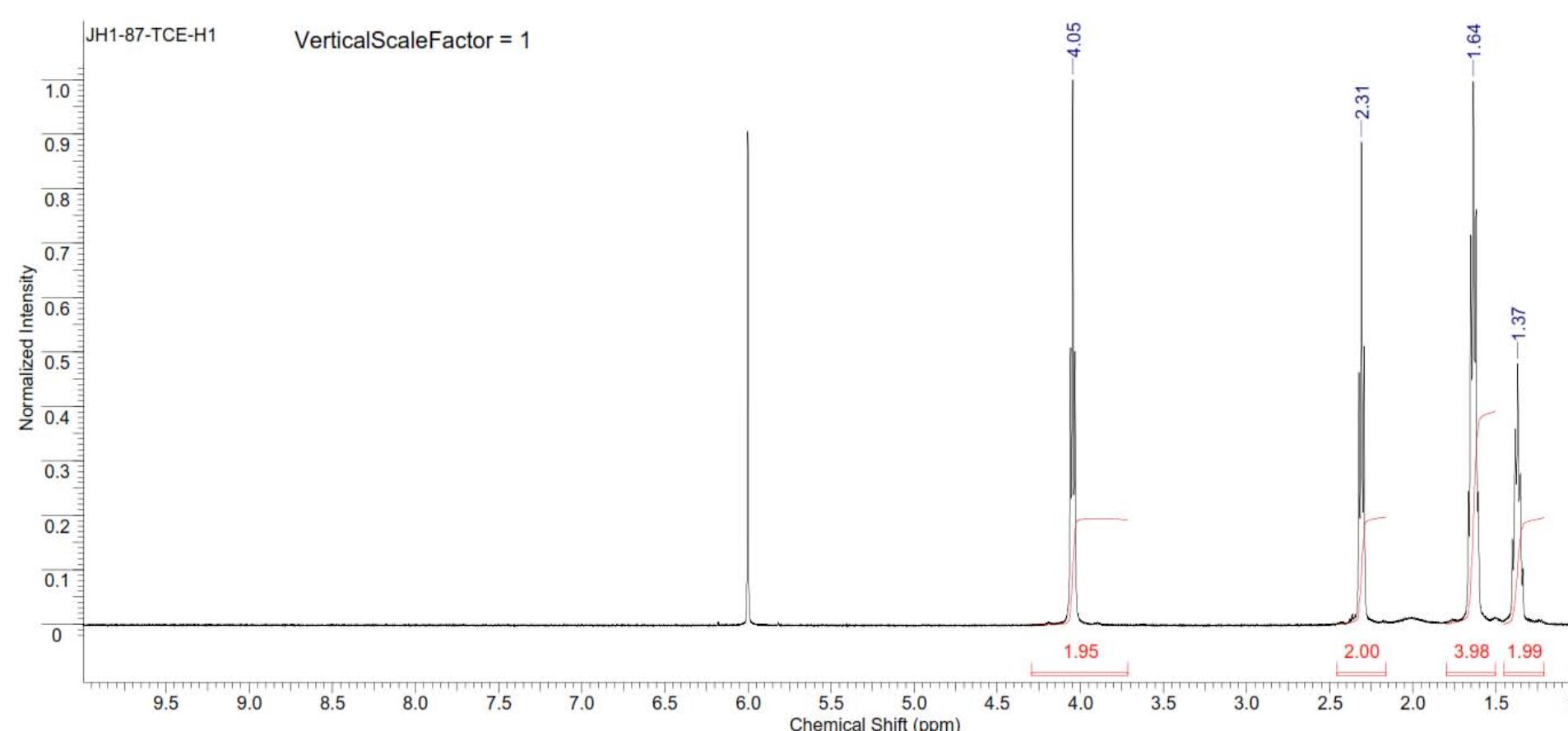
![Figure 8235. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [90:10] (Table S2, entry 2).](https://figures.academia-assets.com/97857879/figure_248.jpg)
![Figure S236. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [80:20] (Table S2, entry 3).](https://figures.academia-assets.com/97857879/figure_249.jpg)
![i al i | Figure $237. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [70:30] (Table $2, entry 4).](https://figures.academia-assets.com/97857879/figure_250.jpg)
![Figure 8238. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [60:40] (Table S2, entry 5).](https://figures.academia-assets.com/97857879/figure_251.jpg)

![Figure $239. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] (Table S2, entry 6).](https://figures.academia-assets.com/97857879/figure_253.jpg)
![ee eT a Dee ee SU ee Figure $240. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [40:30] (Table S2, entry 7).](https://figures.academia-assets.com/97857879/figure_254.jpg)


![ee a we ne ee ee Figure $242. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table 82, entry 9). Figure $241. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [30:70] (Table S2, entry 8).](https://figures.academia-assets.com/97857879/figure_257.jpg)
![Figure $243. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [10:90] (Table S2, entry 10).](https://figures.academia-assets.com/97857879/figure_258.jpg)


![Figure $245. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [90:10] (Table S2, entry 12). $132](https://figures.academia-assets.com/97857879/figure_261.jpg)
![Figure S246. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [80:20] (Table S2, entry 13).](https://figures.academia-assets.com/97857879/figure_262.jpg)
![a ae Figure $247. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [70:30] (Table S2, entry 14).](https://figures.academia-assets.com/97857879/figure_263.jpg)
![Figure $248. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [60:40] (Table S2, entry 15).](https://figures.academia-assets.com/97857879/figure_264.jpg)
![Figure S249. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [50:50] (Table S2, entry 16).](https://figures.academia-assets.com/97857879/figure_265.jpg)
![Figure $250. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [40:60] (Table S2, entry 17).](https://figures.academia-assets.com/97857879/figure_266.jpg)

![Figure $251. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [30:70] (Table S2, entry 18)](https://figures.academia-assets.com/97857879/figure_268.jpg)
![Figure $252. 'd NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [20:80] (Table S2, entry 19).](https://figures.academia-assets.com/97857879/figure_269.jpg)
![Figure $253. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [10:90] (Table S2, entry 20).](https://figures.academia-assets.com/97857879/figure_270.jpg)
![Figure $254. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [90:10] (Table S2, entry 22).](https://figures.academia-assets.com/97857879/figure_271.jpg)
![Figure S255. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [80:20] (Table S2, entry 23).](https://figures.academia-assets.com/97857879/figure_272.jpg)
![Figure $256. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [70:30] (Table S2, entry 24).](https://figures.academia-assets.com/97857879/figure_273.jpg)

![POT ENCSES me OPES Apres Figure $257. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [60:40] (Table S2, entry 25).](https://figures.academia-assets.com/97857879/figure_275.jpg)
![‘igure $258. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [50:50] (Table S2, entry 26).](https://figures.academia-assets.com/97857879/figure_276.jpg)
![Figure $259. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [40:60] (Table S2, entry 27).](https://figures.academia-assets.com/97857879/figure_277.jpg)
![Figure S260. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [30:70] (Table S2, entry 28).](https://figures.academia-assets.com/97857879/figure_278.jpg)
![Figure $261. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [20:80] (Table S2, entry 29).](https://figures.academia-assets.com/97857879/figure_279.jpg)
![Figure $262. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [10:90] (Table S2, entry 30).](https://figures.academia-assets.com/97857879/figure_280.jpg)

![Figure $264. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylcoumaric acid) [80:20] (Table S2, entry 32).](https://figures.academia-assets.com/97857879/figure_282.jpg)
![Figure $265. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylcoumaric acid) [50:50] (Table S2, entry 33).](https://figures.academia-assets.com/97857879/figure_283.jpg)
![Figure $266. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylcoumaric acid) [20:80] (Table S2, entry 34).](https://figures.academia-assets.com/97857879/figure_284.jpg)


![Figure S270. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [60:40] (Table S3, entry 3).](https://figures.academia-assets.com/97857879/figure_287.jpg)
![Figure $271. 'd NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [40:60] (Table S3, entry 4)](https://figures.academia-assets.com/97857879/figure_288.jpg)
![Figure S272. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [20:80] (Table $3, entry 5)](https://figures.academia-assets.com/97857879/figure_289.jpg)
![Ee ee eee ee Ee Figure S273. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [80:20] (Table S3, entry 7).](https://figures.academia-assets.com/97857879/figure_290.jpg)
![Figure $274. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [60:40] (Table S3, entry 8).](https://figures.academia-assets.com/97857879/figure_291.jpg)
![Figure $275. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [40:60] (Table $3, entry 9).](https://figures.academia-assets.com/97857879/figure_292.jpg)
![Figure $276. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [20:80] (Table $3, entry 10).](https://figures.academia-assets.com/97857879/figure_293.jpg)
![Figure $277. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [90:10] (Table $3, entry 12).](https://figures.academia-assets.com/97857879/figure_294.jpg)
![Figure S278. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [80:20] (Table $3, entry 13).](https://figures.academia-assets.com/97857879/figure_295.jpg)
![NSE TSEC TINEES COU TEER A Epee ely Figure S279. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [70:30] (Table S3, entry 14).](https://figures.academia-assets.com/97857879/figure_296.jpg)
![Figure S280. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [60:40] (Table $3, entry 15).](https://figures.academia-assets.com/97857879/figure_297.jpg)
![Figure $281. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [20:80] (Table $3, entry 16).](https://figures.academia-assets.com/97857879/figure_298.jpg)
![Figure $282. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [90:10] (Table S3, entry 18).](https://figures.academia-assets.com/97857879/figure_299.jpg)
![Figure $283. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [80:20] (Table $3, entry 19).](https://figures.academia-assets.com/97857879/figure_300.jpg)
![Figure S284. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [70:30] (Table S3, entry 20).](https://figures.academia-assets.com/97857879/figure_301.jpg)

![a eee ee ee ee Figure $285. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [60:40] (Table S3, entry 21).](https://figures.academia-assets.com/97857879/figure_303.jpg)
![Figure S286. 'H NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [20:80] (Table S3, entry 22).](https://figures.academia-assets.com/97857879/figure_304.jpg)
![Figure S287. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 min. (Table S4, entry 1).](https://figures.academia-assets.com/97857879/figure_305.jpg)
![Figure $288. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 20 min. (Table S4, entry 2).](https://figures.academia-assets.com/97857879/figure_306.jpg)
![Figure $289. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 30 min. (Table S4, entry 3).](https://figures.academia-assets.com/97857879/figure_307.jpg)
![Figure 8290. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1 hour (Table S4, entry 4).](https://figures.academia-assets.com/97857879/figure_308.jpg)
![Figure $291. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1.5 hours (Table S4, entry 5).](https://figures.academia-assets.com/97857879/figure_309.jpg)
![Figure S292. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2 hours (Table S4, entry 6)](https://figures.academia-assets.com/97857879/figure_310.jpg)
![Figure S293. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2.5 hours (Table S4, entry 7 C1412](https://figures.academia-assets.com/97857879/figure_311.jpg)
![Figure $294. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3 hours (Table S4, entry 8).](https://figures.academia-assets.com/97857879/figure_312.jpg)
![Figure $295. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3.5 hours (Table S4, entry 9).](https://figures.academia-assets.com/97857879/figure_313.jpg)
![Figure $296. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4 hours (Table S4, entry 10). $149](https://figures.academia-assets.com/97857879/figure_314.jpg)
![Figure 8297. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4.5 hours (Table S4, entry 11).](https://figures.academia-assets.com/97857879/figure_315.jpg)
![Figure $298. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 5 hours (Table S4, entry 12).](https://figures.academia-assets.com/97857879/figure_316.jpg)
![Figure S299. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 6 hours (Table S4, entry 13).](https://figures.academia-assets.com/97857879/figure_317.jpg)
![Figure $300. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 7 hours (Table S4, entry 14).](https://figures.academia-assets.com/97857879/figure_318.jpg)
![Figure $301. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 8 hours (Table S4, entry 15). g](https://figures.academia-assets.com/97857879/figure_319.jpg)
![Figure S302. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 9 hours (Table S4, entry 16).](https://figures.academia-assets.com/97857879/figure_320.jpg)
![Figure $303. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 hours (Table S4, entry 17).](https://figures.academia-assets.com/97857879/figure_321.jpg)
![Figure $304. 'H NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 13 hours (Table $4, entry 18).](https://figures.academia-assets.com/97857879/figure_322.jpg)






![Figure $311. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [80:20] (Table S2, entry 3). Figure $310. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [90:10] (Table S2, entry 2).](https://figures.academia-assets.com/97857879/figure_329.jpg)
![Figure $312. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [70:30] (Table S2, entry 4).](https://figures.academia-assets.com/97857879/figure_330.jpg)


![Figure $313. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [60:40] (Table S2, entry 5).](https://figures.academia-assets.com/97857879/figure_333.jpg)
![Figure $315. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [40:60] (Table S2, entry 7) Figure S314. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] (Table S2, entry 6).](https://figures.academia-assets.com/97857879/figure_334.jpg)

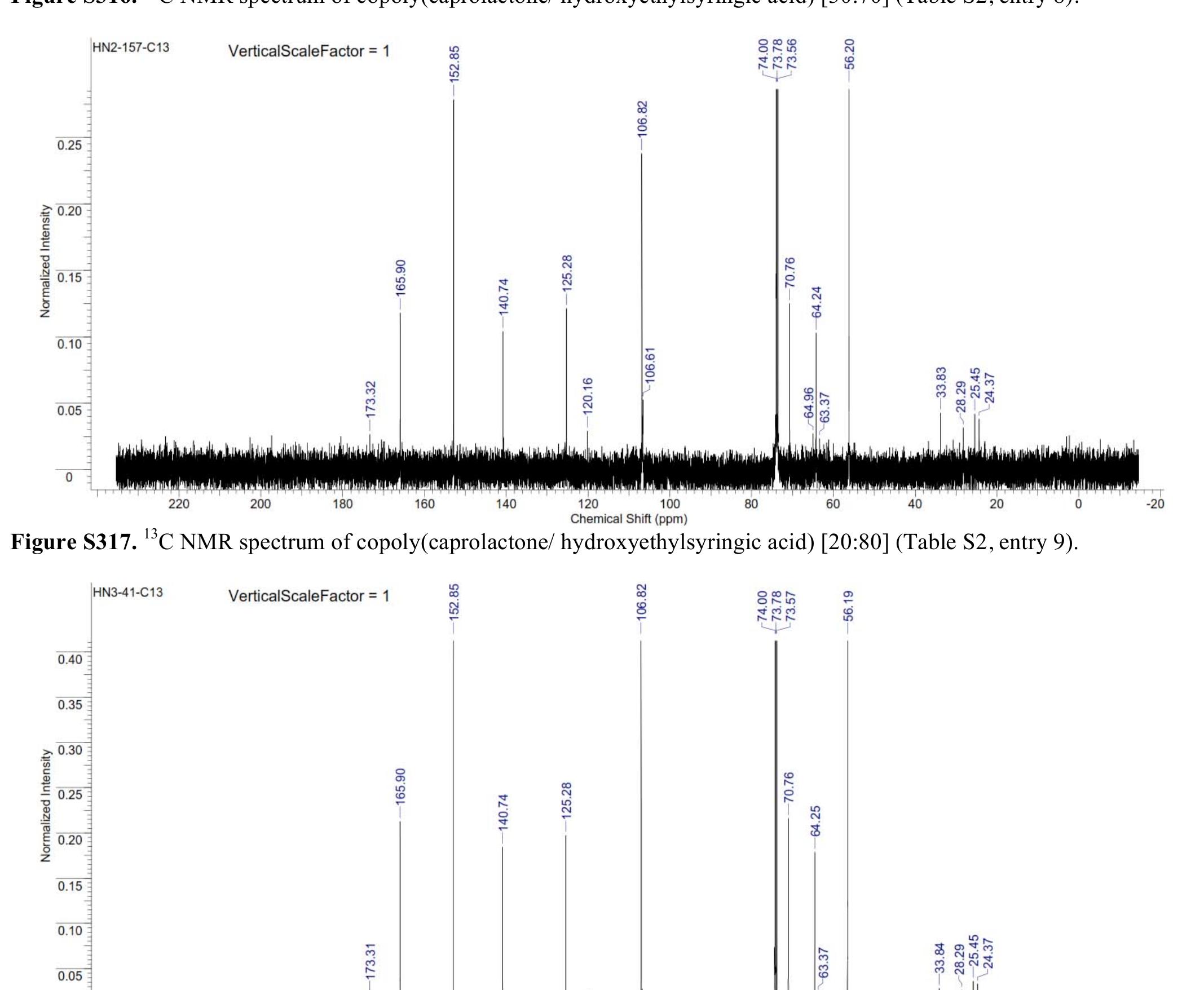
![Figure $316. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [30:70] (Table S2, entry 8).](https://figures.academia-assets.com/97857879/figure_337.jpg)
![Figure $318. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [10:90] (Table S2, entry 10). Figure $317. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [20:80] (Table S2, entry 9).](https://figures.academia-assets.com/97857879/figure_338.jpg)


![Figure $320. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [90:10] (Table S3, entry 12).](https://figures.academia-assets.com/97857879/figure_341.jpg)
![Figure S321. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [80:20] (Table S2, entry 13).](https://figures.academia-assets.com/97857879/figure_342.jpg)
![Figure $322. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [70:30] (Table S2, entry 14).](https://figures.academia-assets.com/97857879/figure_343.jpg)
![Figure $323. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [60:40] (Table S2, entry 15).](https://figures.academia-assets.com/97857879/figure_344.jpg)
![Figure $324. °C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [50:50] (Table S2, entry 16).](https://figures.academia-assets.com/97857879/figure_345.jpg)
![Figure $325. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [40:60] (Table S2, entry 17).](https://figures.academia-assets.com/97857879/figure_346.jpg)
![Figure S326. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylvanillic acid) [30:70] (Table S2, entry 18).](https://figures.academia-assets.com/97857879/figure_347.jpg)

![Figure $327. '°C NMR spectrum of copoly(caprolactone/ hydroxyethyl ferulic acid) [90:10] (Table S2, entry 22).](https://figures.academia-assets.com/97857879/figure_349.jpg)
![Figure $328. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [80:20] (Table S2, entry 23).](https://figures.academia-assets.com/97857879/figure_350.jpg)
![Figure $329. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [70:30] (Table S2, entry 24).](https://figures.academia-assets.com/97857879/figure_351.jpg)
![Figure S330. °C NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [60:40] (Table S2, entry 25).](https://figures.academia-assets.com/97857879/figure_352.jpg)
![Figure $331. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [50:50] (Table S2, entry 26).](https://figures.academia-assets.com/97857879/figure_353.jpg)
![Figure $332. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [40:60] (Table S2, entry 27).](https://figures.academia-assets.com/97857879/figure_354.jpg)
![Figure S333. '*C NMR spectrum of copoly(caprolactone/ hydroxyethylferulic acid) [30:70] (Table S2, entry 28).](https://figures.academia-assets.com/97857879/figure_355.jpg)

![Figure $334. '°C NMR spectrum of polyethylene ferulate (Table S2, entry 31). Figure $335. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylcoumaric acid) [80:20] (Table S2, entry 32).](https://figures.academia-assets.com/97857879/figure_357.jpg)
![Figure S336. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylcoumaric acid) [50:50] (Table S2, entry 33).](https://figures.academia-assets.com/97857879/figure_358.jpg)



![Figure $339. °C NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [80:20] (Table $3, entry 2).](https://figures.academia-assets.com/97857879/figure_362.jpg)
![Figure $340. °C NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [60:40] (Table S3, entry 3).](https://figures.academia-assets.com/97857879/figure_363.jpg)
![Figure S341. '°C NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [40:60] (Table S3, entry 4).](https://figures.academia-assets.com/97857879/figure_364.jpg)
![Figure $342. '°C NMR spectrum of copoly(L-lactide/ hydroxyethylsyringic acid) [20:80] (Table S3, entry 5).](https://figures.academia-assets.com/97857879/figure_365.jpg)
![Figure S343. °C NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [80:20] (Table $3, entry 7).](https://figures.academia-assets.com/97857879/figure_366.jpg)
![Figure $344. °C NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [60:40] (Table S3, entry 8).](https://figures.academia-assets.com/97857879/figure_367.jpg)
![Figure $345. '°C NMR spectrum of copoly(L-lactide/ hydroxyethylvanillic acid) [40:60] (Table S3, entry 9).](https://figures.academia-assets.com/97857879/figure_368.jpg)
![Figure S346. '°C NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [90:10] (Table S3, entry 12).](https://figures.academia-assets.com/97857879/figure_369.jpg)
![Figure $347. °C NMR spectrum of c copoly(L-lactide/ hydroxyethylferulic acid) [80:20] (Table $3, entry 13).](https://figures.academia-assets.com/97857879/figure_370.jpg)
![Figure $348. °C NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [70:30] (Table $3, entry 14).](https://figures.academia-assets.com/97857879/figure_371.jpg)
![Figure $349. °C NMR spectrum of copoly(L-lactide/ hydroxyethylferulic acid) [60:40] (Table $3, entry 15).](https://figures.academia-assets.com/97857879/figure_372.jpg)
![Figure $350. °C NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [90:10] (Table S3, entry 18).](https://figures.academia-assets.com/97857879/figure_373.jpg)
![Figure $351. '°C NMR spectrum of c copoly(L-lactide/ hydroxyethyleoumaric acid) [80:20] (Table S3, entry 19).](https://figures.academia-assets.com/97857879/figure_374.jpg)
![Figure S352. '°C NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [70:30] (Table S3, entry 20).](https://figures.academia-assets.com/97857879/figure_375.jpg)
![Figure S353. °C NMR spectrum of copoly(L-lactide/ hydroxyethylcoumaric acid) [60:40] (Table 3, entry 21).](https://figures.academia-assets.com/97857879/figure_376.jpg)
![Figure $354. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 min. (Table S4, entry 1).](https://figures.academia-assets.com/97857879/figure_377.jpg)
![Figure S355. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 20 min. (Table S4, entry 2).](https://figures.academia-assets.com/97857879/figure_378.jpg)
![Figure $356. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 30 min. (Table $4, entry 3)](https://figures.academia-assets.com/97857879/figure_379.jpg)
![Figure $357. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1 hour (Table S4, entry 4).](https://figures.academia-assets.com/97857879/figure_380.jpg)
![Figure $358. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 1.5 hours (Table S4, entry 5).](https://figures.academia-assets.com/97857879/figure_381.jpg)
![Figure $359. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2 hours (Table S4, entry 6). 0171 ‘igure S359. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2 hours (Table S4, entry 6).](https://figures.academia-assets.com/97857879/figure_382.jpg)
![Figure $360. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 2.5 hours (Table S4, entry 7).](https://figures.academia-assets.com/97857879/figure_383.jpg)
![Figure $361. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3 hours (Table S4, entry 8).](https://figures.academia-assets.com/97857879/figure_384.jpg)
![Figure S362. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 3.5 hours (Table S4, entr 9).](https://figures.academia-assets.com/97857879/figure_385.jpg)
![Figure $363. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4 hours (Table S4, entry 10).](https://figures.academia-assets.com/97857879/figure_386.jpg)
![Figure $364. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 4.5 hours (Table S4, entry 11).](https://figures.academia-assets.com/97857879/figure_387.jpg)
![Figure S365. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 5 hours (Table S4, entry 12).](https://figures.academia-assets.com/97857879/figure_388.jpg)
![Figure S366. '*C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 6 hours (Table S4, entry 13).](https://figures.academia-assets.com/97857879/figure_389.jpg)
![Figure $367. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 7 hours (Table S4, entry 14).](https://figures.academia-assets.com/97857879/figure_390.jpg)

![Figure S368. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 8 hours (Table S4, entry 15).](https://figures.academia-assets.com/97857879/figure_392.jpg)
![Figure $369. '°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 9 hours (Table S4, entry 16).](https://figures.academia-assets.com/97857879/figure_393.jpg)
![Figure S370. °C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 10 hours (Table S4, entry 17).](https://figures.academia-assets.com/97857879/figure_394.jpg)
![Figure $371. ‘°C NMR spectrum of copoly(caprolactone/ hydroxyethylsyringic acid) [50:50] at 13 hours (Table S4, entry 10\](https://figures.academia-assets.com/97857879/figure_395.jpg)










![Figure S380. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 10 min. (Table S4, entry 1)](https://figures.academia-assets.com/97857879/figure_404.jpg)
![Figure S381. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 20 min. (Table S4, entry 2)](https://figures.academia-assets.com/97857879/figure_405.jpg)
![Figure $382. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 30 min. (Table S4, entry 3)](https://figures.academia-assets.com/97857879/figure_406.jpg)
![Figure $383. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 1 hour (Table S4, entry 4)](https://figures.academia-assets.com/97857879/figure_407.jpg)
![Figure S384. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 1.5 hours (Table S4, entry 5)](https://figures.academia-assets.com/97857879/figure_408.jpg)
![Figure S385. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 2 hours (Table S4, entry 6)](https://figures.academia-assets.com/97857879/figure_409.jpg)
![Figure $386. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 2.5 hours (Table S4, entry 7)](https://figures.academia-assets.com/97857879/figure_410.jpg)
![Figure $387. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 3 hours (Table S4, entry 8)](https://figures.academia-assets.com/97857879/figure_411.jpg)
![Figure S388. ESI spectra of copoly(caprolactone/hydroxyethylsyringic acid) [50:50] at 3.5 hours (Table S4, entry 9)](https://figures.academia-assets.com/97857879/figure_412.jpg)

Related papers
Biomacromolecules, 2003
Aliphatic polyesters prepared by ring-opening polymerization of lactones are now used worldwide as bioresorbabale devices in surgery (orthopaedic devices, sutures, stents, tissue engineering, and adhesion barriers) and in pharmacology (control drug delivery). This review presents the various methods of the synthesis of polyesters and tailoring the properties by proper control of molecular weight, composition, and architecture so as to meet the stringent requirements of devices in the medical field. The effect of structure on properties and degradation has been discussed. The applications of these polymers in the biomedical field are described in detail.
Macromolecules, 2006
Metal-free catalysis was successfully applied to polymerize -pentadecalactone (PDL) by ring-opening polymerization (ROP) using several amino-ended initiators, namely hexylamine, allylamine and O, O′-bis(3-aminopropyl)diethylene glycol. This polymerization method was suitable to prepare telechelic polyesters carrying functional-end groups. The technique was then extended to the synthesis of block copolymers by ROP of PDL using bisamino-ended poly(ethylene glycol) (M n =2600) as macroinitiator. PPDL x -PEG 56 -PPDL x triblock copolymers with M n ranging between ~4000 and ~90000 g•mol -1 were synthesized and extensively characterized by NMR, DSC, TGA and XRD. The amphiphilic copolymers thus produced were demonstrated to be able to self-assemble in nanoparticles with average diameters of ~100-200 nm and morphologies highly depending on blocks lengths. The described synthetic route distinguishes in providing "clean" amphiphilic copolymers, which are attractive candidates for biomedical applications.
Chemical Reviews, 2004
2017
The aim of this work was the study of several polymerization systems using lactones as monomers, such as, Ɛ-caprolactone and ɣ-butyrolactone, by a ring opening mechanism. The experiments were performed with acidic catalysts such as, methanesulfonic acid and triflic acid. As indicated in the literature, the ring opening polymerizations with these types of catalysts and ɣ-lactones were not possible under the operating conditions due to the thermodynamic stability of this type of monomer. Thus, to obtain an efficient system, the copolymerization of the two monomers, Ɛ-caprolactone and ɣ-butyrolactone, was performed at different temperatures (-40 °C to 30 °C) and with the two catalysts mentioned above. Novel copolymers, γbutyrolactone-co-Ɛ-caprolactone were obtained at all tested temperatures showing the incorporation of ɣbutyrolactone as desired.
Polymers for Advanced Technologies, 2007
ABSTRACT ABA triblock copolymers of L-lactide (LL) and ε-caprolactone (CL), designated as PLL-P(LL-co-CL)-PLL, were synthesized via a two-step ring-opening polymerization in bulk using diethylene glycol and stannous octoate as the initiating system. In the first-step reaction, an approximately 50:50 mol% P(LL-co-CL) random copolymer (prepolymer) was prepared as the middle (B) block. This was then chain extended in the second-step reaction by terminal block polymerization with more L-lactide. The percentage yields of the triblock copolymers were in excess of 95%. The prepolymers and triblock copolymers were characterized using a combination of dilute-solution viscometry, gel permeation chromatography (GPC), 1H- and 13C-NMR, and differential scanning calorimetry (DSC). It was found that the molecular weight of the prepolymer was controlled primarily by the diethylene glycol concentration. All of the triblock copolymers had molecular weights higher than their respective prepolymers. 13C-NMR analysis confirmed that the prepolymers contained at least some random character and that the triblock copolymers consisted of additional terminal PLL end (A) blocks. From their DSC curves, the triblock copolymers were seen to be semi-crystalline in morphology. Their glass transition, solid-state crystallization, and melting temperature ranges, together with their heats of melting, all increased as the PLL end (A) block length increased. Copyright © 2005 John Wiley &amp; Sons, Ltd.
Journal of Polymer Science Part A: Polymer Chemistry, 2012
A series of di-and triblock copolymers [poly(L-lactide-b-e-caprolactone), poly(D,L-lactide-b-e-caprolactone), poly (e-caprolactone-b-L-lactide), and poly(e-caprolactone-b-L-lactideb-e-caprolactone)] have been synthesized successfully by sequential ring-opening polymerization of e-caprolactone (e-CL) and lactide (LA) either by initiating PCL block growth with living PLA chain end or vice versa using titanium complexes supported by aminodiol ligands as initiators. Poly(trimethylene carbonate-b-e-caprolactone) was also prepared. A series of random copolymers with different comonomer composition were also synthesized in solution and bulk of e-CL and D,L-lactide. The chemical composition and microstructure of the copoly-mers suggest a random distribution with short average sequence length of both the LA and e-CL. Transesterification reactions played a key role in the redistribution of monomer sequence and the chain microstructures. Differential scanning calorimetry analysis of the copolymer also evidenced the random structure of the copolymer with a unique T g .
Macromolecules
Statistical copolymers of L-lactide (L-LA) and ε-caprolactone (CL) are of major interest as a result of the desired combination of properties they exhibit for high-added-value applications, including in the biomedical field and in microelectronics. However, the high difference of reactivity between the two monomers makes difficult their statistical insertion in copolymer chains. Here, the ring-opening polymerization and copolymerization (ROP and ROcP, respectively) of L-LA and CL mediated by benzoic acid (BA) are investigated by means of density functional theory (DFT). It is first evidenced that the mechanism involves a hydrogen-bonding dual activation, where the acidic proton of BA activates the carbonyl moiety of the monomer, while the conjugated base of BA activates the alcohol initiator. In accordance with experimental findings, DFT calculations have then revealed a kinetically favored energetic profile for the BA-organocatalyzed ROP of CL compared to L-LA. In addition, energetic profiles of the BA-mediated ROcP of CL and L-LA does not show any preference of the insertion between CL and L-LA, irrespective of the type of growing species. Even though the caproyl unit insertion is kinetically favored by the primary nature of the growing chain end alcohol, this is eventually mitigated by the stabilizing effect of the ester moieties of the lactidyl unit, which is thermodynamically favored. As one effect compensates for the other, the dual activation mechanism involved in this organocatalytic pathway using BA as a weak organic acid is shown to be crucial to achieve truly statistical copolymers based on L-LA and CL.
2014
This project analyzed the ring opening chemistry of D, L-lactide, γ-butyrolactone, valerolactone, dodecalactone and caprolactone. Starting with each of the above monomers, Sn(Oct)2,SnCl2, Zn(acac)2, ZnCL2, and AlCl3 were used as catalysts in the polymerization process. Initiators included benzyl alcohol, 2-phenylethanol and 1-butanol. The results of each reaction were analyzed by 1H-NMR and IR spectroscopy and dynamic light scattering (DLS). The results were collated to determine the most promising candidates for a student project in the teaching laboratory.
International Journal of Biological Macromolecules, 1999
Lipase catalysis induced a ring-opening polymerization of lactones with different ring-sizes. Small-size (four-membered) and medium-size lactones (six-and seven-membered) as well as macrolides (12-, 13-, 16-, and 17-membered) were subjected to lipase-catalyzed polymerization. The polymerization behaviors depended primarily on the lipase origin and the monomer structure. The macrolides showing much lower anionic polymerizability were enzymatically polymerized faster than o-caprolactone. The granular immobilized lipase derived from Candida antartica showed extremely efficient catalysis in the polymerization of o-caprolactone. Single-step terminal functionalization of the polyester was achieved by initiator and terminator methods. The enzymatic polymerizability of lactones was quantitatively evaluated by Michaelis-Menten kinetics.
The Journal of Organic Chemistry, 2009
The thermodynamics of polymerization of ε-caprolactone and 1,4-dioxan-2-one has been investigated theoretically and compared with that recently reported for δ-valerolactone and γ-butyrolactone. Specifically, the ability of these monomers to polymerize has been related to the strain of the rings, the Gibbs free energy of simple models for ring-opening reactions of the cyclic lactones, and the conformational preferences of linear model compounds of the corresponding homopolyesters. The results are fully consistent with the lack of polymerizability of γ-butyrolactone, while the ring openings of ε-caprolactone and δ-valerolactone have been found to be exergonic processes. Polymerizability of 1,4-dioxan-2-one has been found to be favored, even though less than that of ε-caprolactone and δ-valerolactone. Two factors explain these features: (i) the strain of the ester group in the lactones increases with the exergonic character of the ring-opening process, and (ii) the stability of coiled conformations in model compounds follows this order: poly-4-hydroxybutyrate > poly(1,4-dioxan-2-one) > poly-6-hydroxycaproate ≈ poly-5-hydroxyvalerate. Finally, the influence of the environment on the polymerizability of the three cyclic lactones is discussed in detail.
Macromolecular Bioscience, 2004
Polymer Chemistry, 2013
Linear trimethylene carbonate (1,3-dioxane-2-one, TMC) thermoplastic copolymers derived from glycerol have been synthesized upon sequential copolymerization. The "immortal" ring-opening polymerization (iROP) of the benzyloxy-substituted TMC, 3-benzyloxytrimethylene carbonate (BTMC), with systems composed of the b-diketiminate discrete zinc complex [(BDI iPr )Zn(N(SiMe 3 ) 2 )] or the phosphazene base 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP) as a catalyst, and benzyl alcohol (BnOH) as an initiator/chain transfer agent is first described. Next, diblock and triblock copolycarbonates featuring a TMC segment along with one or two adjacent BTMC, or 2,2dimethoxypropane-1,3-diol carbonate (TMC(OMe) 2 ), or racemic b-butyrolactone (BL) chains have been prepared. The copolymerization under "immortal" operating conditions was carried out in bulk or toluene solution and promoted by [(BDI iPr )Zn(N(SiMe 3 ) 2 )] as a catalyst, and either BnOH, 1,3-propanediol, or the poly(trimethylene carbonate) (PTMC) derived macro-ols, H-PTMC-OBn or H-PTMC-H, as an initiator/chain transfer agent. In particular, the PTMC-b-[poly(TMC(OMe) 2 )] 1,2 couple of copolymers provide a basis for the assessment of thermal and mechanical property differentiation in close relationship with the co-monomers content. Unlike PTMC which is elastomeric and P(TMC(OMe) 2 ) which is rather rigid and brittle much like semi-crystalline poly(L-lactide), the diblock and triblock copolymers show characteristics lying in between those of each homopolymer, without any significant influence of the topology. Thus PTMC/PTMC(OMe) 2 block copolymers mechanically behave similarly to PTMC/PLLA block copolymers.
Journal of Polymer Science Part A: Polymer Chemistry, 2011
Repeating sequence copolymers of poly(lactic-co-caprolactic acid) (PLCA), poly(glycolic-co-caprolactic acid) (PGCA), and poly(lactic-co-glycolic-co-caprolactic acid) (PLGCA) have been synthesized by polymerizing segmers with a known sequence in yields of 50-85% with M n s ranging from 18-49 kDa. The copolymers exhibited well-resolved NMR resonances indicating that the sequence encoded in the segmers used in their preparation is retained and that transesterification is minimal. The exact sequences allowed for unambiguous assignment of the NMR spectra, and these standards were compared with the data previ-ously reported for random copolymers. The glass transition temperatures (T g s) of the PLCA and PGCA copolymers were found to depend primarily on monomer ratio rather than sequence. Sequence dependent T g s were, however, noted for the PLGCA polymers with 1:1:1 L:G:C ratios; poly LGC and poly GLC exhibited T g s that differed by nearly 8 C.
Macromolecules, 2002
The kinetics of bulk polymerization of 6-, 7-, 9-, 12-, 13-, 16-, and 17-membered lactones initiated with a zinc 2-ethylhexanoate/butyl alcohol system at 100°C was studied and compared with that of lipase-catalyzed polymerization. Instantaneous concentrations of the lactone monomers were determined on the basis of the relative intensities of signals in the 1 H NMR spectra (500 MHz, CDCl3 as a solvent, room temperature) from the ω-methylene protons (-(CH2)x-1CH2OC(O)-) (where x ) 4, 5, 7, 10, 11, 14, and 15) in the lactone monomer and the polyester repeating units, respectively. Linearity of the semilogarithmic kinetic dependencies (ln([lactone]0/[lactone]) vs time), revealed a first order of propagation in monomer for all of the polymerizations studied. This kinetic behavior, pointing to the constant concentration of the involved active centers and thus to the practical elimination of termination side reaction, allowed the relative polymerization rates to be determined. The following order of polymerization rates has been obtained: 2500:330:21:0.9:1.0:0.9:1.0 for the 6-, 7-, 9-, 12-, 13-, 16-, and 17-membered lactones, respectively. The order of rates of the enzymatic polymerization, determined earlier in an independent paper, shows an inverted dependence on the ring size, namely 0.10:0.13:0.19:0.74:1.0 for the 7-, 12-, 13-, 16-, and 17-membered lactones, respectively. The resulting difference in the orders of lactone reactivities in chemical and enzymatic polymerizations is explained in terms of a difference in factors controlling polymerization rates in both processes. The ring strain, which decreases with increasing lactone size, is partially released in the transition state of the elementary reaction of the polyester chain growth, which eventually leads to faster propagation for more strained monomers in chemical polymerizations. In enzymatic polymerizations, the rate-determining step involves formation of the lactone-lipase complex. The latter reaction is promoted by the hydrophobicity of the lactone monomer, which is higher for the larger lactone rings.
Journal of Polymer Science Part A: Polymer Chemistry, 2016
Polyamides (PA) constitute one of the most important classes of polymeric materials and have gained strong position in different areas, such as: textiles, fibers and construction materials. Whereas most polyamides are synthesized by step-growth polycondensation, polyamide 6 is synthesized by ring opening polymerization (ROP) of ε-caprolactam. The most popular ROP methods involve the use of alkaline metal catalyst difficult to handle at large scale. In this article, we propose the use of organic acids for the ROP of ε-caprolactam in bulk at 180 °C (below the polymer's melting point). Among evaluated organic acids, sulfonic acids were found to be the most effective for the polymerization of ε-caprolactam, being the Brønsted acid ionic liquid: 1-(4-sulfobutyl)-3-methylimidazolium hydrogen sulfate the most suitable due to its higher thermal stability. End-group analysis by 1 H nuclear magnetic resonance (NMR) and model reactions provided mechanistic insights and suggested that the catalytic activity of sulfonic acids was a function of not only the acid strength, but of the nucleophilic character of conjugate base as well. Finally, the ability of sulfonic acid to promote the copolymerization of ε-caprolactam and ε-caprolactone is demonstrated. As a result, poly(εcaprolactam-co-ε-caprolactone) copolymers with considerably randomness are obtained. This benign route allows the synthesis of poly(ester amide)s with different thermal and mechanical properties.
Journal of the Mechanical Behavior of Biomedical Materials, 2012
Polymer Degradation and Stability - POLYM DEGRAD STABIL, 1998
Conditions of the living homopolymerization of ε-caprolactone (CL), lactides (LA), and of the homo-oligomerization of γ-butyrolactone (BL) are briefly described. Then block and random copolymerizations of CL with LA are shortly reviewed. The microstructure of the resulting copolyesters in relation to some peculiarities of these processes is discussed in more detail. It is also shown that the otherwise ‘non-polymerizable’ BL does form high molecular weight copolymers with CL, containing up to 50 mol% repeating units derived from BL. Their molecular weight is controlled by the concentrations of the consumed comonomers and the starting concentration of the initiator. NMR and DSC data indicate the random structure of copolymers. TGA traces of the BL/CL copolymers show that the presence of the γ-oxybutyryl repeating units randomly distributed within the poly(CL) chains improves the thermal stability of the latter.
Polymers for Advanced Technologies, 2002
Numerous cyclic dibutyltin alkoxides were prepared by condensation of Bu 2 Sn(OMe) 2 with various short or long a,!-diols. Insertion of lactones or lactide into the SnÐO bonds resulted in ring-expansion polymerizations which allowed a control of the ring size (chain length) via the monomer initiator ratio. When the cyclic initiators were derived from a long a,!-diol, such as poly(tetrahydrofuran)diols or polysiloxane diols, the resulting cyclic polylactones were necessarily cyclic triblock copolymers. The high nucleophilicity of the Sn-O bond enabled ringopening polycondensations with dicarboxylic acid dichlorides yielding multiblock copolyesters. Condensations with monocarboxylic acids yielded functionalized A-B-A triblock copolymers. Polycondensation with trifunctional acid chlorides yielded biodegradable networks. Hydroxyethylated pentaerythritol condensed with Bu 2 Sn(OMe) 2 yielded a spirocyclic initiator. Ringexpansion polymerization with lactones followed by acylation with carboxylic acid chlorides produces starshaped polylactones having functional endgroups. Biodegradable networks were also obtained when bisstannylenated a-glucose methyl glycoside was used as initiator for e-caprolactone, and when the resulting spirocyclic polylactone was polycondensed with sebacoyl chloride.
Polym. Chem., 2015
The successful ring-opening copolymerization of ethylene carbonate (EC) with various cyclic esters such as β-butyrolactone (BL), δ-valerolactone (VL), ε-caprolactone (CL) or l-lactide (LLA) has been achieved.
Related topics
Cited by
Polymers
Plastics are perceived as modern and versatile materials, but their use is linked to numerous environmental issues as their production is based on finite raw materials (petroleum or natural gas). Additionally, their low biodegradability results in the accumulation of microplastics. As a result, there is extensive interest in the production of new, environmentally friendly, bio-based and biodegradable polymers. In this context, poly(ethylene vanillate) (PEV) has a great potential as a potentially bio-based alternative to poly(ethylene terephthalate); however, it has not yet been extensively studied. In the present work, the preparation of PEV is reported. The enthalpy and the entropy of fusion of the pure crystalline PEV have been estimated for the first time. Additionally, the equilibrium melting temperature has also been calculated. Furthermore, the isothermal and non-isothermal crystallization behavior are reported in detail, and new insights on the thermal stability and degradati...
Green Chemistry
What are the most promising biobased PET replacements? Are they economically feasible? Are they sustainable? Industrially feasible? In the future, PET will certainly be replaced by more than one option, e.g., PEF, PTF, bio-PET, and PLA.
Current Organic Synthesis
Recent advances in the application of environmentally benign acid catalysts in organic synthesis are reviewed. The work includes three main parts; (i) description of environmentally benign acid catalysts, (ii) synthesis with heterogeneous and (iii) homogeneous catalysts. The first part provides a brief overview of acid catalysts, both solid acids (metal oxides, zeolites, clays, ion-exchange resins, metal-organic framework based catalysts) and those that are soluble in green solvents (water, alcohols) and at the same time could be regenerated after reactions (metal triflates, heteropoly acids, acidic organocatalysts etc.). The synthesis sections review a broad array of the most common and practical reactions such as Friedel-Crafts and related reactions (acylation, alkylations, hydroxyalkylations, halogenations, nitrations etc.), multicomponent reactions, rearrangements and ring transformations (cyclizations, ring opening). Both the heterogeneous and homogeneous catalytic synthesis pa...
International Journal of Molecular Sciences, 2022
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY
Polymers, 2021
Enzymatic synthesis of aromatic biobased polyesters is a recent and rapidly expanding research field. However, the direct lipase-catalyzed synthesis of polyesters from ferulic acid has not yet been reported. In this work, various ferulic-based monomers were considered for their capability to undergo CALB-catalyzed polymerization. After conversion into diesters of different lengths, the CALB-catalyzed polymerization of these monomers with 1,4-butanediol resulted in short oligomers with a DPn up to 5. Hydrogenation of the double bond resulted in monomers allowing obtaining polyesters of higher molar masses with DPn up to 58 and Mw up to 33,100 g·mol−1. These polyesters presented good thermal resistance up to 350 °C and Tg up to 7 °C. Reduction of the ferulic-based diesters into diols allowed preserving the double bond and synthesizing polyesters with a DPn up to 19 and Mw up to 15,500 g·mol−1 and higher Tg (up to 21 °C). Thus, this study has shown that the monomer hydrogenation strate...
Scientific Reports, 2020
Coral reefs are vital for the marine ecosystem and their potential disappearance can have unequivocal consequences on our environment. Aside from pollution-related threats (changes in water temperature, plastics, and acidity), corals can be injured by diseases, predators, humans and other invasive species. Diseases play an important role in this decline, but so far very few mitigation strategies have been proposed and developed to control this threat. In this work, we demonstrate that recently developed bi-layer human skin wound treatment patches containing antiseptics and natural antioxidants with controlled-release capacity can be adapted to treat scleractinian coral wounds effectively. A hydrophilic bilayer film based on polyvinylpyrrolidone (PVP) and hyaluronic acid was used to cover the open wounds while delivering the antiseptics for rapid action. Afterwards, the hydrophilic bi-layer covered wound was sealed with an antioxidant and hydrophobic ε-caprolactone-p-coumaric acid co...
Polymer Chemistry, 2018
Biosuccinic acid, obtainedviasugar fermentation, is cyclodimerized and oxidized to yield building blocks for aromatic polyesters with high glass transition temperatures.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
 Pengxu Qi
Pengxu Qi