Aerobic Copper-Catalyzed Organic Reactions
…
225 pages
1 file

Sign up for access to the world's latest research
AI-generated Abstract
This paper explores the rich chemistry of copper as a catalyst, emphasizing its capability to adopt various oxidation states and catalyze reactions with molecular oxygen. The introduction highlights the advantages of using oxygen as an oxidant, due to its atom-economical and environmentally friendly properties, as well as the associated challenges in reaction conditions. The text discusses alternative approaches to safely utilize oxidation chemistry involving copper catalysts across different environments, including innovative reactor designs and the application of supercritical carbon dioxide.
Figures (629)















































































































![Scheme 122. Arene Functionalization of a Copper(III)— Azacalix[1]arene[3]pyridine Complex](https://figures.academia-assets.com/36433264/figure_104.jpg)






























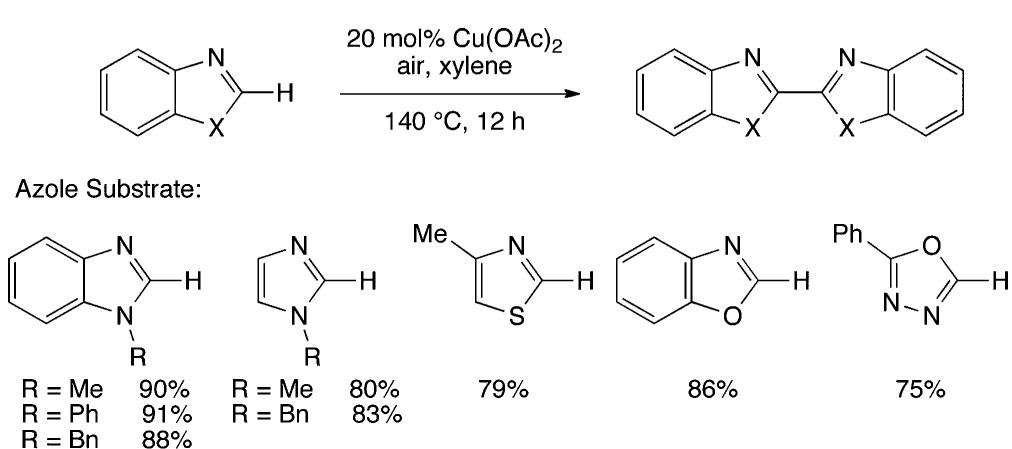
















































































































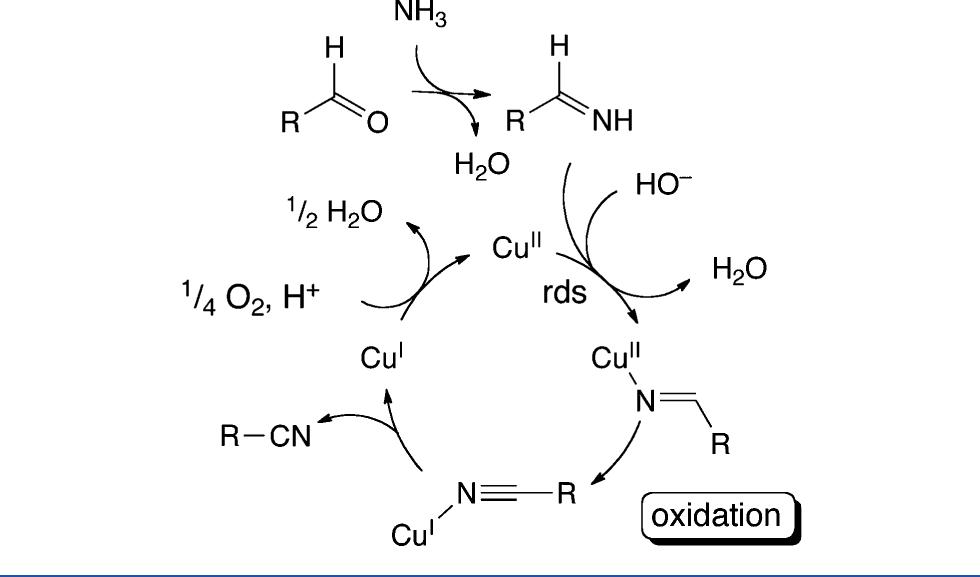

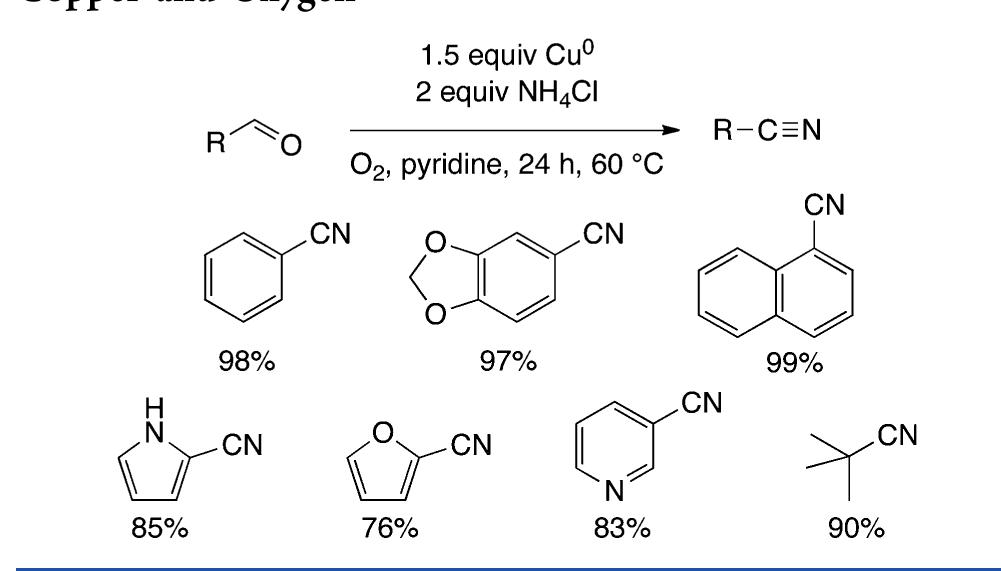

































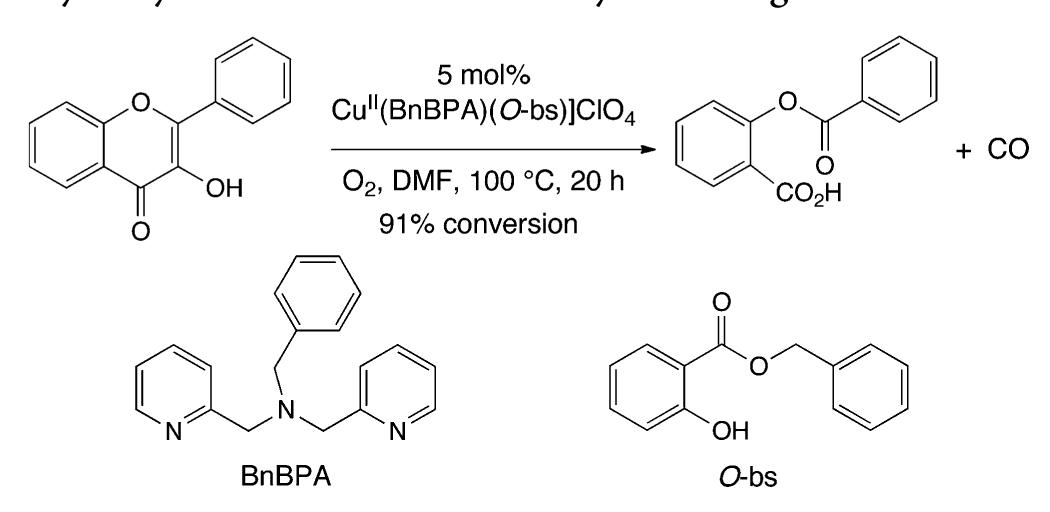









































































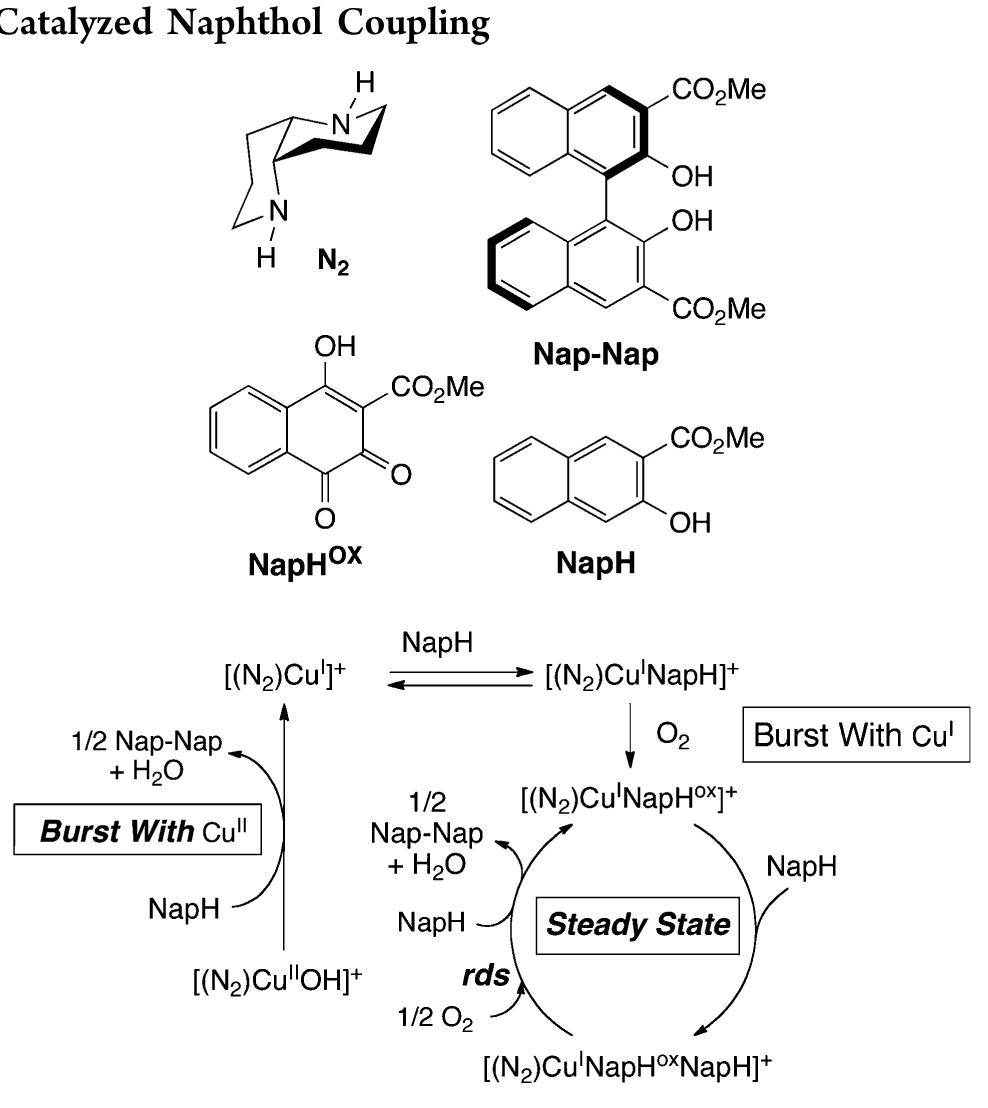



















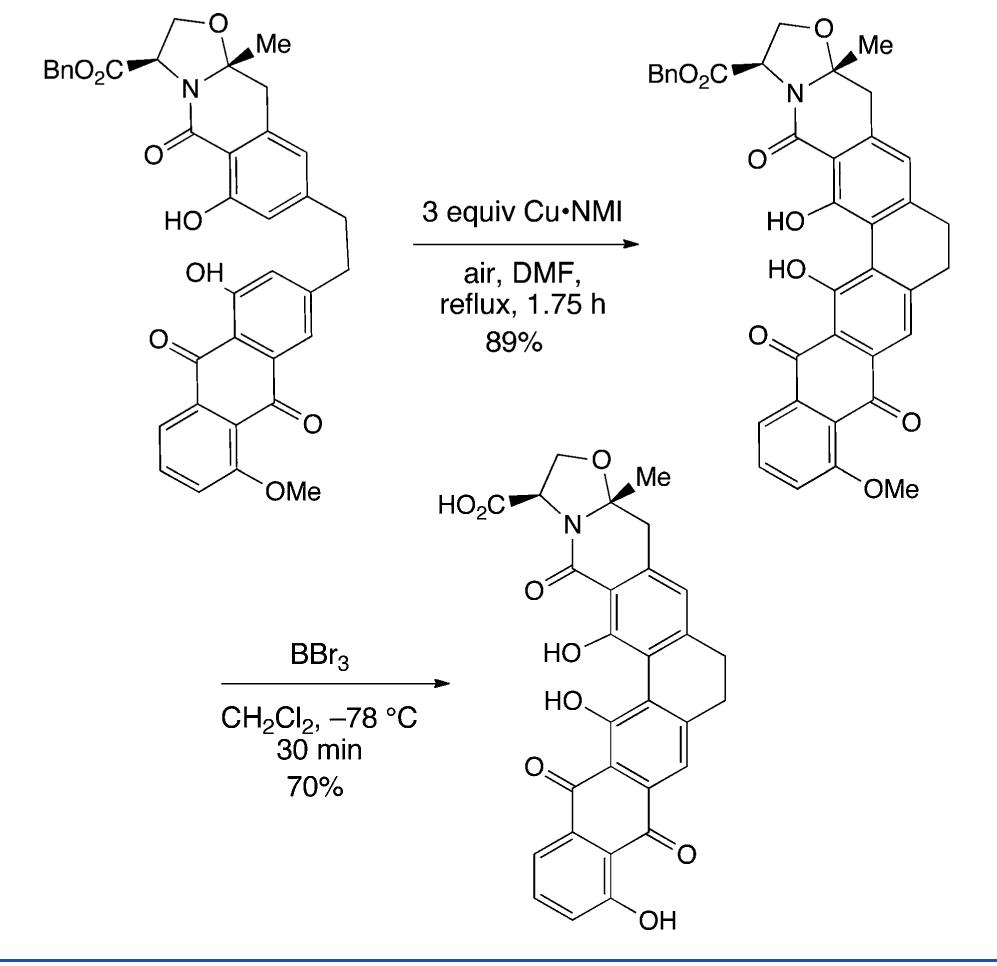





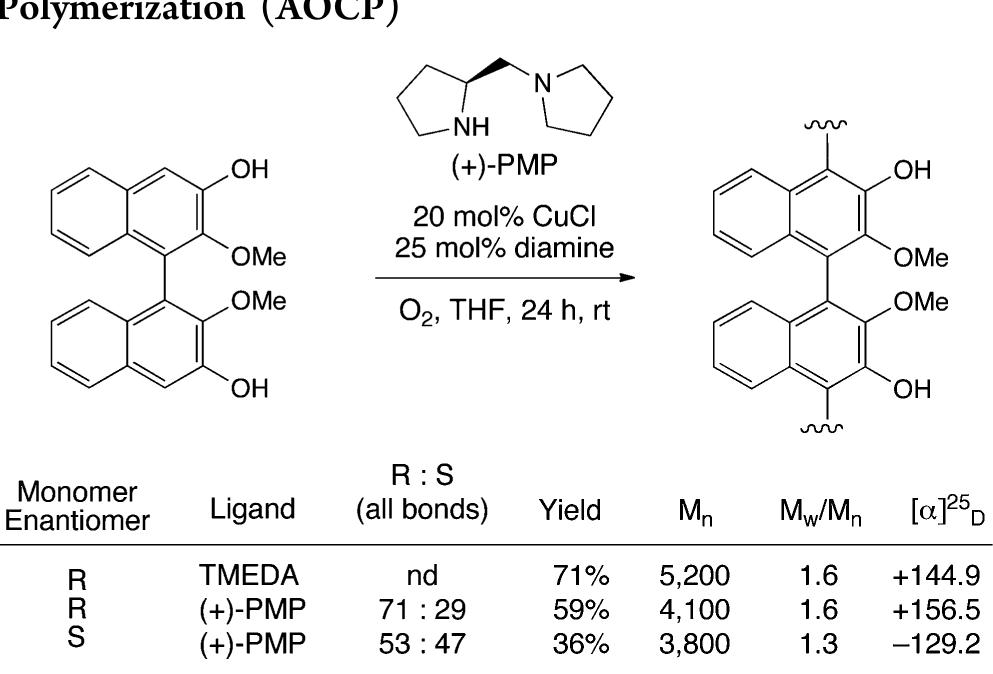



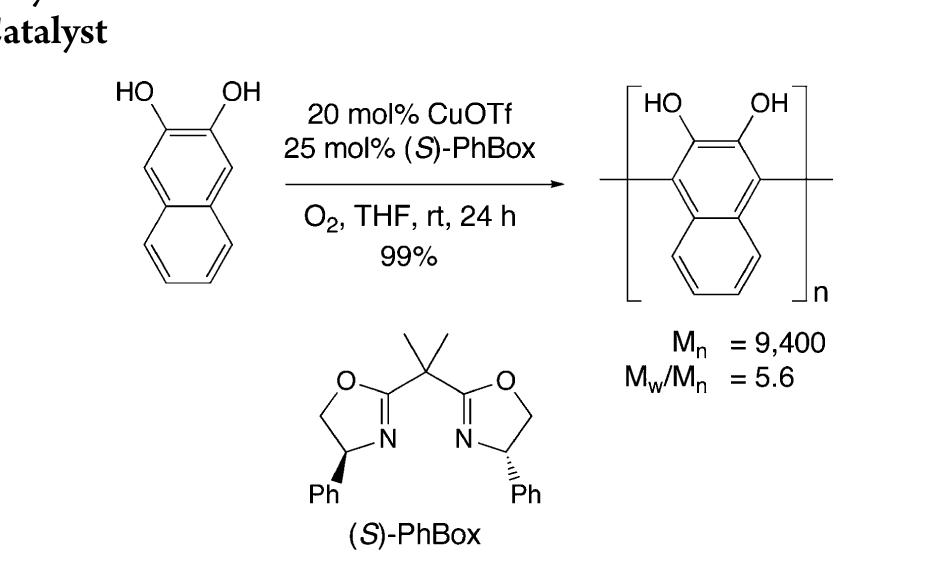















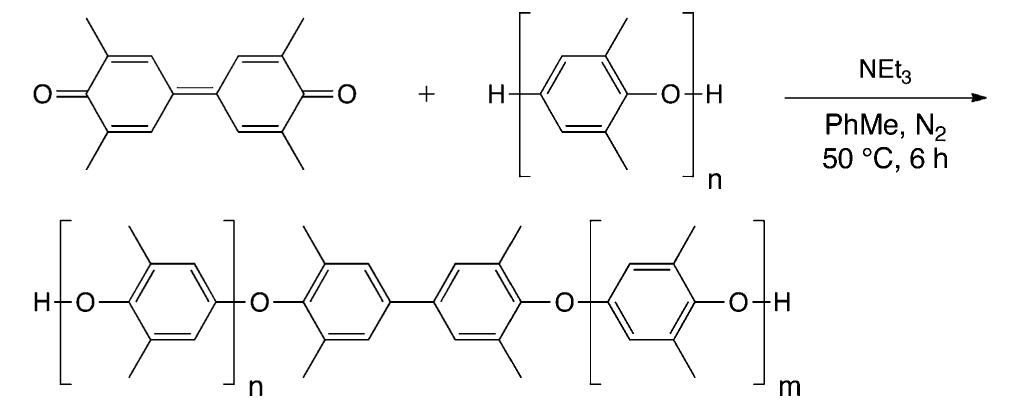













![Scheme 446. Synthesis of 2H-1-Benzopyran-5,8-quinones from para-Methoxyphenol Scheme 447. Mechanism of the Tandem Saucy—Marbet— Claisen, [1,5]-Hydride Shift, and 6-7 Electrocyclization](https://figures.academia-assets.com/36433264/figure_396.jpg)




































































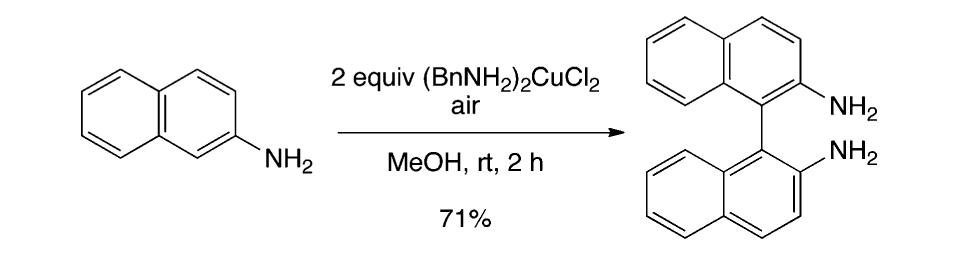






















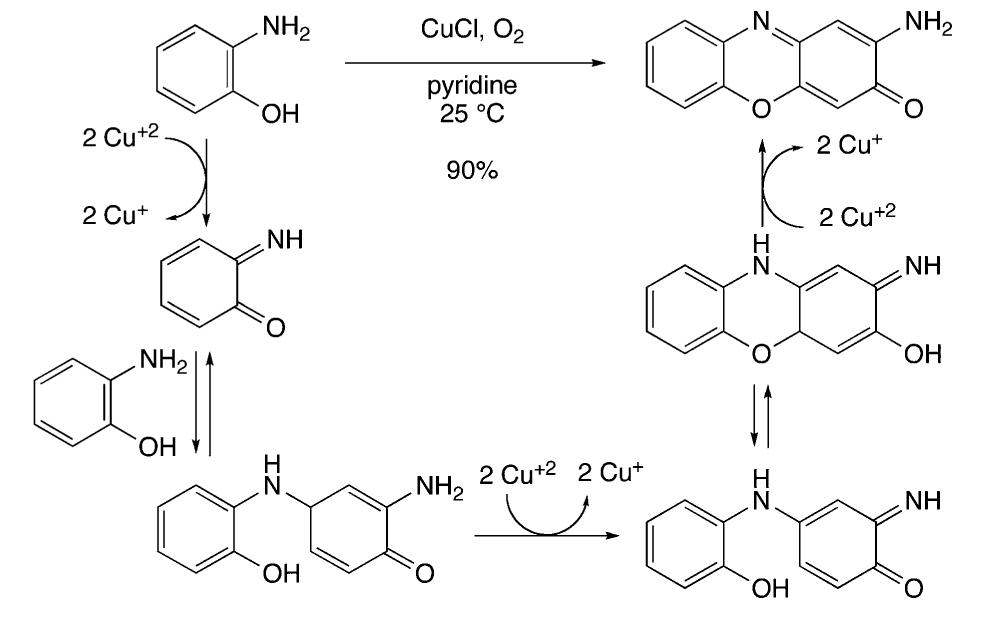
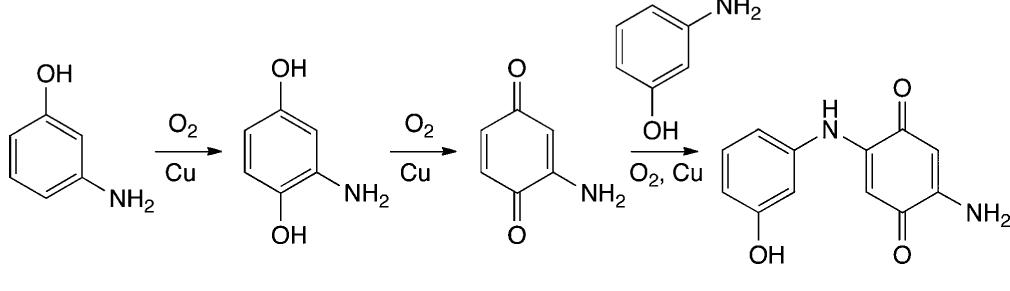
![Scheme 535. Copper-Catalyzed Oxidative Dimerization To Construct Phenazine-Containing Heterocycles Simple anilines were noted to form azo compounds under these conditions (see section 8.2, Scheme 504). However, the absence of azo byproducts here makes a mechanism proceeding through initial N—-N coupling and subsequent [3,3]-sigma- tropic rearrangement unlikely. The reaction is proposed to occur through initial oxidation of the aniline to the radical and dimerization to form the ortho C—N bond. A series of subsequent oxidations via copper can occur with concomitant cyclization to afford the phenazine-containing product. Alternately, the compound may dimerize via an iminoquinone (see Scheme 533). Mechanistic studies are needed to clarify the reaction pathways here.](https://figures.academia-assets.com/36433264/figure_476.jpg)





































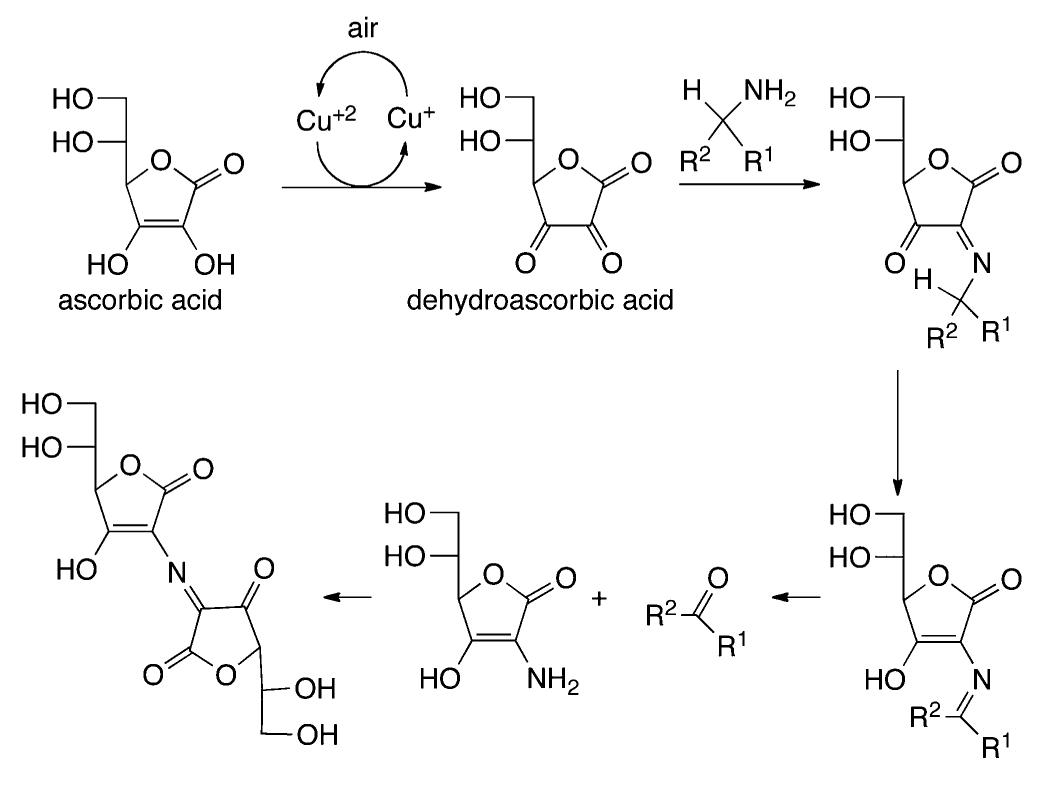







































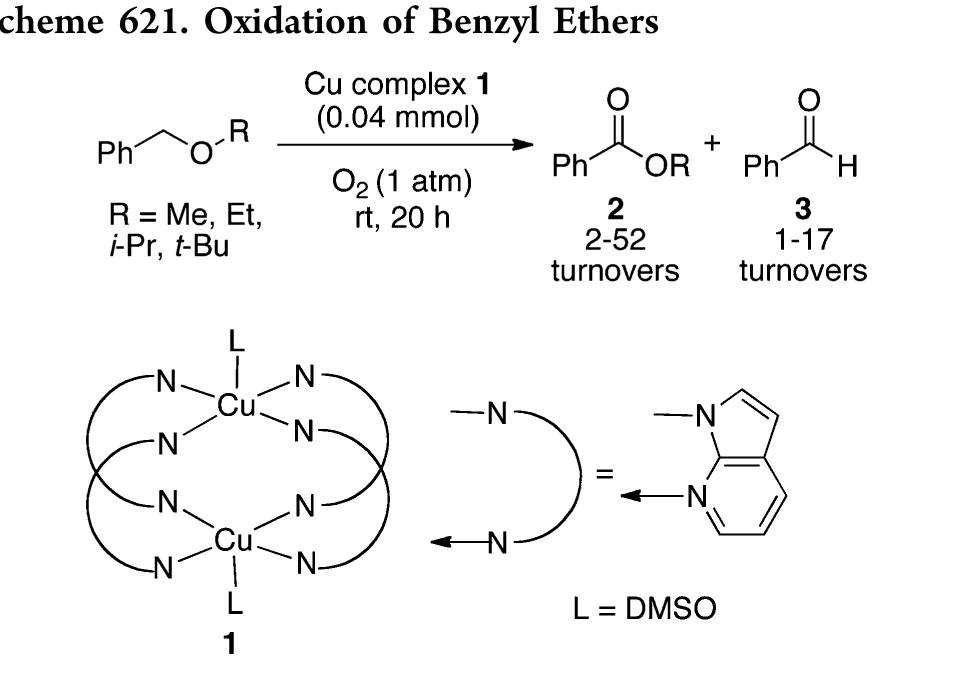
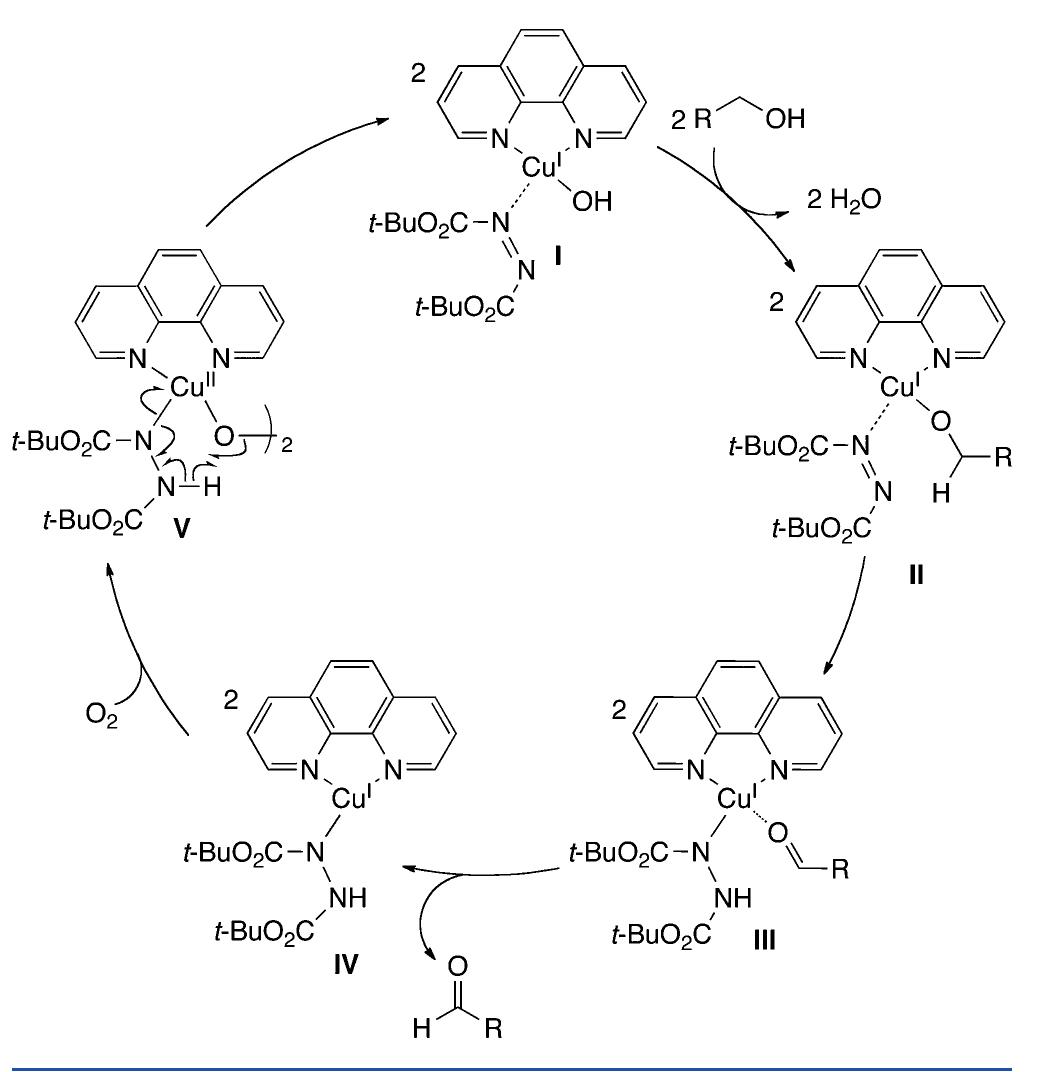
![In an effort to further understand the effects of ligand tuning on the equilibrium of copper bishydroxo species, Zhang and co- workers synthesized several Cu(I) complexes containing tridentate bis[2-(2-pyridyl)ethyl]methyl amine ligands. Varying the electronics of these ligands by modification of the 4- substituent influenced the reactivity of the copper complexes in the two-electron oxidations of tetrahydrofuran, N-methylani- line, and primary alcohols (Scheme 622).''*” Notably, these reactions are not catalytic. Experiments employing 80, indicated that hydroxylation of tetrahydrofuran involves transfer of an oxygen atom from the copper bishydroxo complex.](https://figures.academia-assets.com/36433264/figure_557.jpg)













Key takeaways
- The copper source for this reaction was important because not all copper sources promoted the reaction.
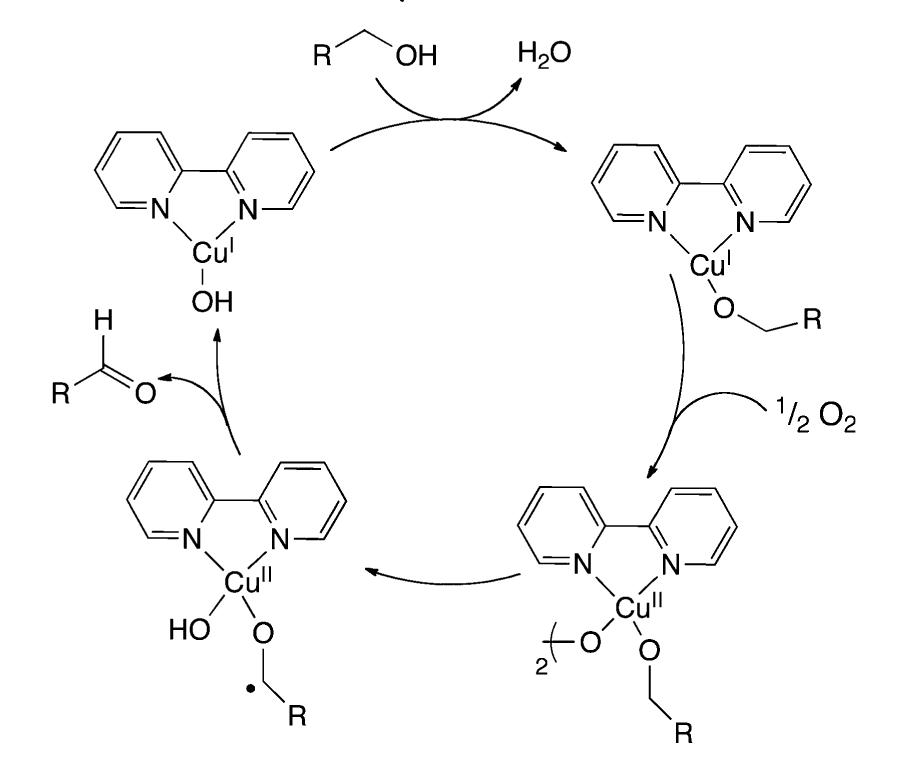
- 512,512, Oxidation to the alcohol is concurrent with reduction of the copper center and is followed by the oxidation of the copper and ligand by molecular oxygen to regenerate the active catalyst.
- Both a copper and oxygen are required for this transformation to occur.
- Later studies showed that the reaction can be performed using excess copper(II) under a nitrogen atmosphere, suggesting that the oxygen is only required to oxidize the copper(I) to copper(II) and that the incorporated oxygen comes from that initial catalyst oxidation.
- However, under these conditions, it is highly probable that much of the copper is in the copper(II) oxidation state.
Related papers
Journal of the American Chemical Society, 2009
Journal of Molecular Catalysis a Chemical, 1996
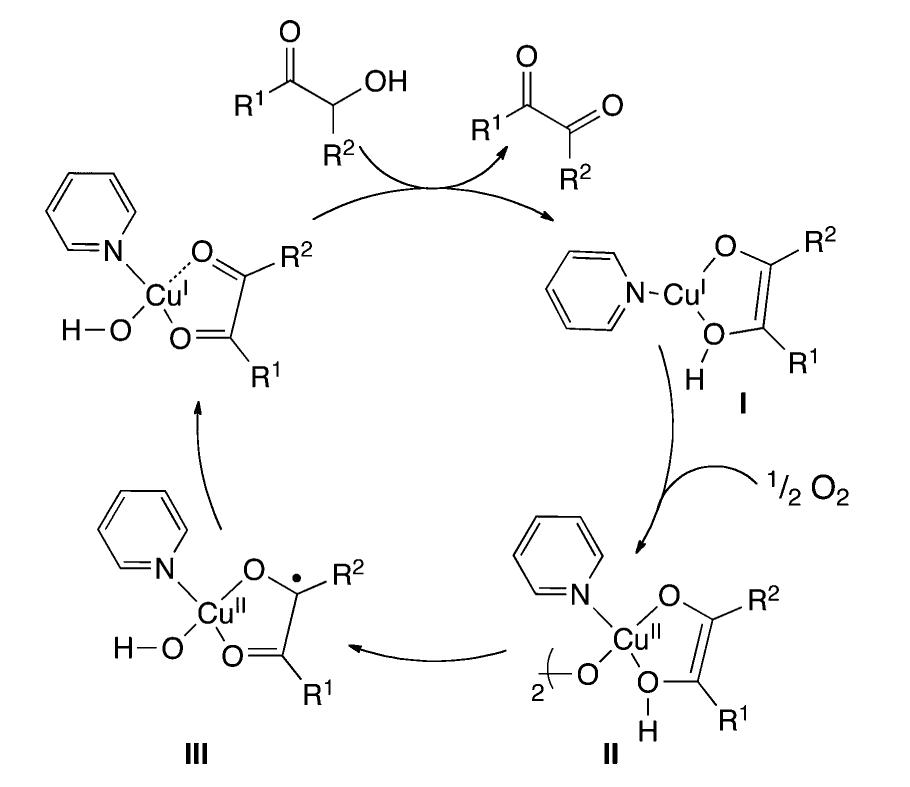
The oxidation of I,?-dials through copper promoted activation of molecular oxygen was studied. The influence of the substituents and experimental conditions is discussed. and examples of catalytic applications are reported.
ChemSusChem, 2008
Selective oxidation of alcohols to aldehydes is a cornerstone transformation for many biological and synthetic organic reactions, providing key biological intermediates and valuable pharmaceuticals. In comparison to the enzymatic oxidation of alcohols, most oxidations in modern organic chemistry are lacking in efficiency and other attributes important in green chemistry, as they often require volatile organic solvents and lead to the generation of stoichiometric amounts of hazardous waste products. The well-established principles favor the use of renewable feedstocks, recyclable catalytic systems, minimum amounts of organic solvents, and low-energy reaction conditions. Although many advances in the design of catalytic aerobic oxidation have been accomplished, a major challenge that persists is that the catalysts developed for aerobic alcohol oxidation are relatively inactive under ambient solventfree conditions. Herein, we report our discovery of a novel three-component catalytic aerobic oxidative system consisting of acetamido-TEMPO (TEMPO = 2,2,6,6-tetramethylpiperidine-Noxide), copper bromide, and 4-pyrrolidinopyridine. This catalytic system yields the highest reported turnover frequencies (up to 200 turnovers per hour) for the ambient chemospecific aerobic oxidation of primary benzylic and allylic alcohols to aldehydes under solvent-free conditions. Continuing on from Sheldon's [CuBr 2 (2,2'-bipyridine)]/TEMPO catalytic system for aerobic alcohol oxidation in a 1:2 water/acetonitrile solvent mixture, our system provides the next generation of copper-TEMPO aerobic oxidation systems, with up to a 14-fold increase in turnover frequency. Furthermore, our studies are accomplished under solvent-free conditions, and the isolation of catalyst-free products and catalyst-recycling can be accomplished by using an antisolvent precipitation methodology.
Journal of Molecular Catalysis A: Chemical, 1997
The oxidation of alkanes to the corresponding alcohols and ketones and the epoxidation of alkenes can be performed efficiently at room temperature with molecular oxygen (1 atm) in the presence of an aldehyde and a copper salt catalyst such as coppe&I) hydroxide. Extremely high turnover numbers have been obtained for the oxidation of cyclohexane using a combination of copper(H) chloride and a crown ether as a catalyst.
Tetrahedron Letters, 1996
Copper-crown ether catalysed oxidation of alkanes with molecular oxygen in the presence of acetaldehyde gives the corresponding ketones and alcohols highly efficiently.
Science, 1996
An efficient, copper-based catalyst has been discovered that oxidizes a wide range of alcohols into aldehydes and ketones under mild conditions. This catalytic system utilizes oxygen or air as the ultimate, stoichiometric oxidant, producing water as the only by-product.
Coordination Chemistry Reviews, 1992
ABSTRACT
Beilstein Journal of Organic Chemistry, 2013
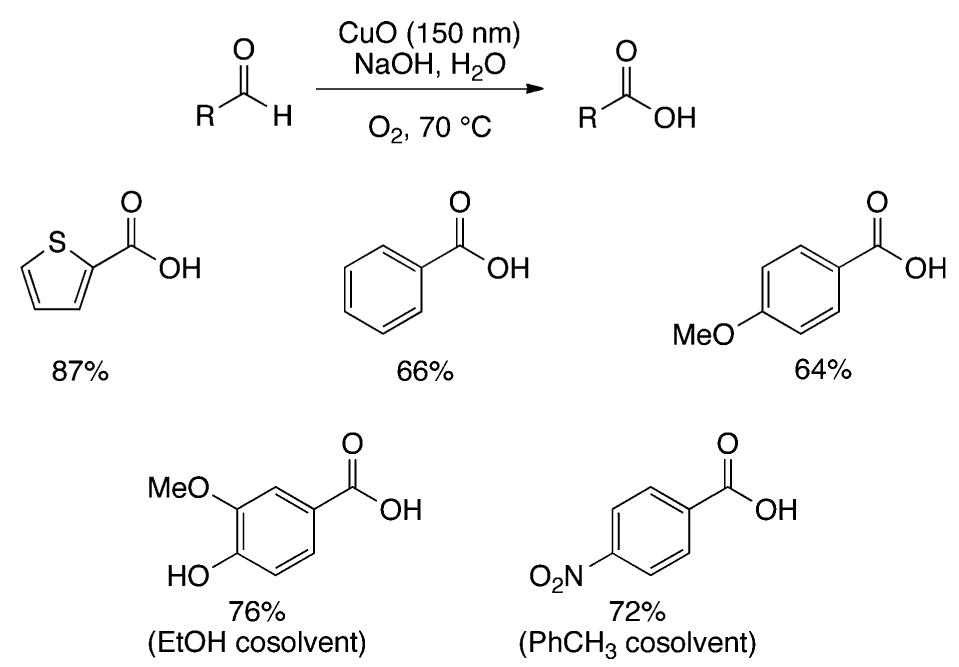
We report herein Cu-catalyzed aerobic oxygenation of aliphatic C-H bonds with hydroperoxides, which proceeds by 1,5-H radical shift of putative oxygen-centered radicals (O-radicals) derived from hydroperoxides followed by trapping of the resulting carboncentered radicals with molecular oxygen.
ChemInform, 1999
The oxidation of allylic and benzylic alcohols to aldehydes can be carried out at room temperature as low as 25°C with molecular oxygen, in the presence of the bifunctional osmium-copper system OsO4-CuC1 acting as the catalyst.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
Related papers
Applied Catalysis A: General, 2012
Environmental technology, 2018
SpringerBriefs in Molecular Science, 2016
Tetrahedron Letters, 2000
Advanced Synthesis and Catalysis, 2017
Environmental science and pollution research international, 2018
Journal of Molecular Catalysis, 1992
Advanced Synthesis & Catalysis, 2004
Pure and Applied Chemistry, 2000
IJC-B Vol.60B(04) [April 2021], 2021
RSC Advances, 2022
Tetrahedron Letters, 1999
Inorganic Chemistry, 1992
Catal. Sci. Technol., 2015
European Journal of Inorganic Chemistry, 2000
European Journal of Inorganic Chemistry
 Maestría Química
Maestría Química